Response
Definitions
Hematologic response (HR)
Complete hematologic response (CHR)
WBC <10 × 109/L
Platelet count <450 × 109/L
Basophil <5%
No immature cells
Spleen nonpalpable
Cytogenetic response (CyR)
Complete cytogenetic response (CCyR)
No Ph + metaphases
Partial cytogenetic response (PCyR)
1–35% Ph + metaphase
Major cytogenetic response (MCyR)
0–35% Ph + metaphase (CCyR + PCyR)
Molecular response
Major molecular response (MMR)
BCR-ABL1 transcript to control transcripts = <0.1% by RQ-PCR (IS) or MR3.0 or better
MR3.0, MR4.0, MR4.5, MR5.0
3, 4, 4.5, 5 log reduction in BCR-ABL1 transcript from the standard baseline
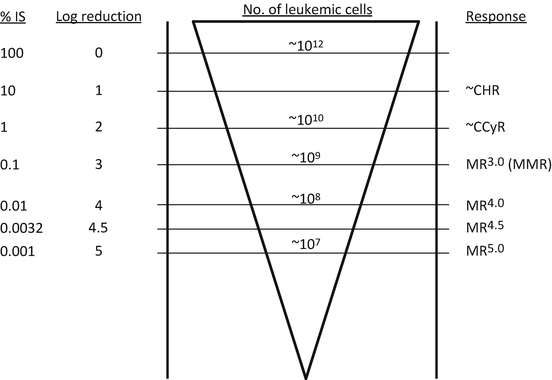
Fig. 3.1
Definitions of response and molecular levels of BCR-ABL1 transcripts. IS international scale, CHR complete hematologic response, CCyR complete cytogenetic response, MR molecular response, MMR major molecular response
3.3 Clinical Trials of Imatinib for CML
3.3.1 Phase 1 Study for CML in CP
A phase 1 dose-escalating study of imatinib was conducted from June 1998 [3]. Imatinib was administered orally once daily to 83 patients with CML-CP who were resistant or showed intolerance to treatment with interferon alfa (IFNα). Imatinib was generally well tolerated, although a maximal tolerated dose was not identified despite dose escalation from 25 to 1000 mg. The most common adverse effects included nausea, myalgia, edema, and diarrhea. At a minimum dose of 300 mg/day, imatinib showed cytogenetic response in 29 of the 54 patients, which included 7 patients with CCyR. Further, 400 mg daily dose inhibited the enzymatic activity of BCR-ABL1 in vivo, as demonstrated by decreased phosphorylation of CRKL, a substrate of BCR-ABL1. Therefore, a daily dose of at least 400 mg was recommended for future studies.
3.3.2 Phase 2 Studies for CML in CP, CML in AP, and CML in BP
In a phase 2 study of patients with CML-CP, a total of 532 patients with late-CP CML who failed to respond to IFNα were treated with 400 mg of imatinib daily [10]. Imatinib induced major cytogenetic response (MCyR) in 60% of the 454 patients and complete hematologic response (CHR) in up to 95% of the patients. Over a median follow-up duration of 18 months, the estimated progression-free survival (PFS) and overall survival (OS) was 89% and 95%, respectively. Further, the estimated PFS and OS at 6 years was 61% and 76%, respectively. Incidence of serious adverse events was <6%; hematologic toxic effects were manageable.
In a phase 2 study of imatinib, patients with newly diagnosed CML-AP or CML-BP were treated with 400 or 600 mg imatinib daily and were evaluated for sustained hematologic response (HR) lasting at least 4 weeks. Among the 181 patients with CML-AP, the rate of HR was 82%, and the rate of sustained HR lasting at least 4 weeks was 69% (CHR 34%) [11]. Rates of MCyR and CCyR were 24% and 17%, respectively. The estimated PFS and OS at 12 months was 59% and 74%, respectively.
In 229 patients with CML in myeloid BP, the rate of HR was 52%, and the rate of sustained HR lasting at least 4 weeks was 31% (CHR 9%) [12]. Rates of MCyR and CCyR were 16% and 7%, respectively. The estimated median response duration was 10 months, and the median survival was 6.9 months. Imatinib at an initial dose of 600 mg was associated with a significant improvement in therapeutic efficacy and survival, over that observed with 400 mg dose in patients with CML-AP and CML-BP.
A Japanese phase 2 study of imatinib (600 mg daily) plus chemotherapy (JALSG ALL202 trial) was conducted for newly diagnosed patients (n = 77) with Ph-positive acute lymphoblastic leukemia (ALL) [13]. The primary end point was complete remission rate. Complete remission was achieved in 96.2%, while CCyR was achieved in 71.3% patients. The median duration of complete remission was 5.2 months. The estimated 1-year EFS and OS rates were 60% and 76.1%, respectively. The outcomes were significantly better than those of historical controls.
3.3.3 Outcomes of Imatinib Treatment in Newly Diagnosed CML-CP
A prospective, multicenter, phase 3, randomized study [International Randomized Study of Interferon and STI571 (IRIS)] involving newly diagnosed patients with CML-CP (n = 1016) was initiated in June 2000 [14]. Patients were randomized to two treatment arms; the imatinib (STI571) group received imatinib 400 mg once daily, and patients in the IFNα + cytarabine group received IFNα (target dose: 5 million units/m2 per day), and subcutaneous low-dose (20 mg/m2 per day) cytarabine was added for 10 days every month. Patients were allowed to cross over to the other arm in the event of a lack of response, a loss of response, or development of intolerance to treatment. The primary end point was event-free survival (EFS). Events were defined by the first occurrence of any of the following: death from any cause during treatment, progression to AP or BP, or loss of CHR or MCyR.
Over a median follow-up duration of 18 months, the estimated MCyR was 87.1% and 34.7% in the imatinib and IFNα + cytarabine groups, respectively (P < 0.001) [14]. The estimated CCyR was 76.2% and 14.5%, respectively (P < 0.001); estimated PFS at 18 months was 96.6% and 79.9%, respectively (P < 0.001). Due to the substantial superiority of imatinib detected on interim analysis, the study results were disclosed early and most patients were crossed over to the imatinib arm. Accordingly, this study is now effectively a long-term follow-up study of patients who received imatinib as the initial therapy. After a median follow-up of 60 months, 382 (69%) of the 553 patients randomized to the imatinib arm remained on imatinib, and only 16 (3%) of the 553 patients randomized to IFNα + cytarabine arm remained on the same regimen [4].
In the imatinib group, the cumulative response rate of CCyR at 12 and 60 months was 69% and 87%, respectively. CyR rate and disease progression showed a significant association with Sokal risk. However, once a CCyR was achieved, it appeared to prevent disease progression even in patients with a higher Sokal risk. The estimated rates of EFS and survival without progression to AP/BP at 60 months were 83% and 93%, respectively. The estimated rates of survival without AP/BP at 60 months were 100% in patients who achieved CCyR with MMR at 12 months and 95% in those who achieved CCyR without MMR (P = 0.007). Annual rates for all events as well as those of progression to AP/BP declined over time with imatinib therapy. The estimated OS at 60 months was 89%; when the analysis was censored for deaths unrelated to CML, OS rate was estimated to be 95% (Fig. 3.2).
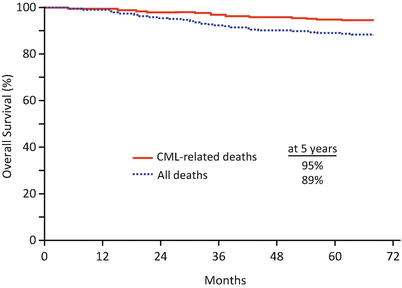

Fig. 3.2
Overall survival in imatinib group in the IRIS trial at a median follow-up of 5 years, on an intention to treat analysis. The estimated 5-year OS (blue line) was 89%, and the estimated 5-year OS with deaths associated with only CML (red line) was 95% in imatinib group (Adapted from Druker et al. [4]. Copyright © 2006 Massachusetts Medical Society. All rights reserved)
In the most recent update of the IRIS trial, the estimated OS at 8 years was 85% [15]. MMR increased from 24% at 6 months to 39% at 12 months and to a best rate of 86% at 8 years, in a total of 98 patients who were sequentially monitored for BCR-ABL1 transcript.
In a phase 2 study involving 204 patients with newly diagnosed CML-CP at the Hammersmith Hospital in the United Kingdom, the cumulative incidence of CCyR and MMR at 5 years was 82.7% and 50.1%, respectively [16]. The estimated 5-year OS and PFS was 83.2% and 82.7%, respectively. Twenty-five percent of patients had discontinued imatinib treatment because of unsatisfactory response and/or toxicity by 5 years of treatment. Patients who achieved CCyR at 1 year had a better PFS and OS than those who failed to reach CCyR; however, achievement of MMR appeared to confer no further advantage.
A prospective multicenter phase 2 study (CML202 trial) of imatinib therapy in newly diagnosed patients with CML-CP was performed in Japan Adult Leukemia Study Group (JALSG) [17]. Over a median follow-up of 65 months, the estimated 7-year OS and EFS in 481 evaluable patients were 93% and 87%, respectively. Cumulative incidence of MMR at 18 months and 7 years from the start of imatinib was 39% and 79%, respectively. Figure 3.3 represents the survivals of newly diagnosed patients with CML before and after imatinib era in JALSG studies [17–19].
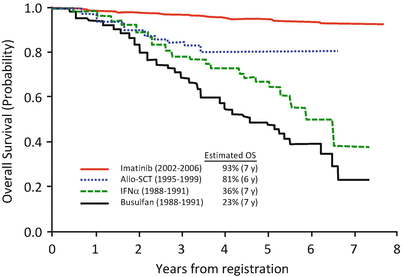

Fig. 3.3
Therapeutic advances in newly diagnosed patients with CML-CP based on JALSG studies. This chart illustrates the survivals of adult patients with newly diagnosed CML-CP who were prospectively enrolled in JALSG studies from 1988 to 2006. From 1988 to 1991, 170 patients were enrolled in a phase 3 study that was conducted to compare the effect of IFNα with that of busulfan. The estimated 7-year rates of OS were 36% in IFNα group (n = 85, green line) and 23% in busulfan group (n = 85, black line), respectively (Adapted from Ohnishi et al. [18]). From 1995 to 1999, 257 patients were enrolled in a prospective study to investigate the optimal indication of SCT. The estimated 6-year OS (blue line) of patients younger than 50 years who received allo-SCT was 81% (n = 82) (Adapted from Ohnishi et al. [19]). From 2002 to 2006, 489 patients were enrolled in CML202 trial to examine the efficacy of imatinib therapy. The estimated 7-year OS (red line) was 93% (Adapted from Ohnishi et al. [17])
In newly diagnosed patients with CML-CP treated with 400 mg imatinib, the reported rates of CCyR and MMR at 1 year ranged from 49% to 77% and from 18% to 58%, respectively [7]. Over a minimum duration of 5 years, PFS ranged between 83% and 94%, and OS ranged between 83% and 97% (Table 3.2). The proportion of patients who continued to receive imatinib treatment at 3–5 years from initiation ranged between 63% and 79%, while 50% were continuing treatment after 8 years. However, differences in the definition of PFS and EFS used in these studies should be taken into account while comparing the long-term outcomes in these studies. In some studies, data on patients who were taken off therapy for reasons other than progression were censored. Kantarjian et al. proposed that any instance of toxicity or death from any cause, on or off therapy, should be counted as an event to calculate EFS and PFS [20].
Table 3.2
Long-term outcomes of first-line treatment of imatinib for newly diagnosed patients with CML-CP in clinical studies
Study/source | Imatinib dose, mg | No. of patients | CCyR % | MMR % | OS % | PFS % | EFS % | At year |
|---|---|---|---|---|---|---|---|---|
Best rate | Best rate | |||||||
400 | 553 | 83 | 86 | 85 | 92 | 81 | 8 | |
Hammersmith hospital [16] | 400 | 204 | 83 | 50 | 83 | 83 | 81a | 5 |
JALSG CML202 [17] | 400b | 481 | 90 | 79 | 93 | 93 | 87 | 7 |
3.4 Adverse Events
The most common adverse events of imatinib 400 mg reported from the IRIS trial [4] and the Japanese CML202 trial [17] were edema (peripheral and periorbital edema) (60% and 49%), muscle cramps (49% and 17%), diarrhea (45% and 16%), nausea (50% and 22%), musculoskeletal pain (47% and 21%), rash (40% and 40%), fatigue (39% and 24%), and headache (37% and 8%), respectively. Incidence of grade 3 or 4 adverse events in both trials was neutropenia (17%, 18%), thrombocytopenia (9%, 12%), anemia (4%, 6%), and elevated liver enzymes (5%, 4%), respectively. Several types of vascular adverse events have been reported in patients receiving second- or third-generation BCR-ABL1 TKIs [21]. However, imatinib exhibits a favorable long-term safety profile. The vascular safety of imatinib therapy is well documented and occurrence of congestive heart failure has been rare (1.7%) [22]. Newly occurring or worsening grade 3 or 4 hematologic or biochemical adverse events were infrequent both after 2 and 4 years of treatment. Imatinib is generally well tolerated; most adverse events were grade 1 or 2 in severity and tended to be most frequent in the first year of treatment. Discontinuation of imatinib for drug-related adverse events was required in <4% of patients over a median follow-up duration of 60 months.
3.5 Dosing Issue
The recommended starting dose of imatinib for patients with CML-CP is 400 mg/day, while that for patients with CML-AP or BP is 600 mg/day. Efficacy evaluation of imatinib 800 mg/day was performed in a phase 3, randomized study (TOPS trial). Newly diagnosed patients with CML-CP were treated with either 800 mg/day or 400 mg/day of imatinib [23, 24]. The primary end point was MMR at 12 months. MMR rates were similar in both treatment arms at 12 months (51.6% vs. 50.2%; P = 0.77) and 42 months (75.8% vs. 79.0%; P = 0.48). Further, no significant between-group difference was observed with respect to EFS, PFS, or OS. However, patients who tolerated more than 600 mg/day of imatinib showed a higher response rate.
In the ELN study, 216 patients with newly diagnosed CML at high Sokal risk were randomly assigned to receive imatinib 800 or 400 mg/day for at least 1 year [25]. The primary end point of CCyR at 12 months was comparable between the two treatment arms, as were the OS, PFS, and EFS. In the high-dose arm, the median average daily dose was 720 mg (range: 350–800 mg).
In a phase 2 study (TIDEL trial) of higher-dose imatinib, patients with newly diagnosed CML-CP initiated on imatinib 600 mg/day were shifted to 800 mg/day, if the prespecified response criteria were not met [26]. The primary end points were MMR, MCyR, and CCyR at 2 years. Both TIDEL trial and IRIS trial showed comparable MMR rates at 12 months (47% and 40%, respectively). However, the MMR at 24 months was higher in TIDEL trial (73% vs. 55% in the IRIS trial). Superior response in patients who were able to tolerate imatinib at 600 mg suggests that early dose intensity may be critical to achieve optimal response in CML-CP.
Overall, the current body of evidence alone does not justify extensive use of higher-dose imatinib (800 mg daily) as a frontline therapy in patients with CML-CP. Indeed, treatment adherence appears to be more important than initiation of treatment at a higher dose.
Subgroup analyses of a Japanese phase 2 study (CML202 trial) were performed according to the mean daily dose during the first 24 months of treatment: ≥360 mg (400 mg group; n = 294), 270–359 mg (300 mg group; n = 90) and <270 mg (200 mg group; n = 67). This was because imatinib dosage was reduced in many patients due mainly to adverse events [17]. No significant between-group differences were observed in OS and EFS between the 300- and 400-mg groups. These results indicate that long-term outcomes in small, elderly, and/or female patients who received 300 mg imatinib daily were similar to those who received 400 mg/day. However, cumulative rates of cytogenetic (92% vs. 98%; P = 0.018) or molecular response (78% vs. 87%, P = 0.017) at 7 years in the 300-mg group were inferior to those in the 400-mg group. Further, survival and efficacy was markedly inferior in the 200-mg group. Therefore, excessive dose reduction (<300 mg/day) should be avoided even in patients who are intolerant to imatinib 400 mg/day or in those who have a small body size.
3.6 Imatinib and Interferon α Combination
IFNα can induce sustained cytogenetic response in some patients with CML-CP [27]. Furthermore, Essers et al. reported that acute stimulation with IFNα activates dormant hematopoietic stem cells, while chronic stimulation leads to their exhaustion [28]. In a German CML Study IV, a randomized 5-arm trial designed to optimize imatinib therapy, 465 patients were randomized to imatinib alone or imatinib plus IFNα 2a or 2b [29]. Imatinib + IFNα failed to show superiority over imatinib monotherapy with respect to MMR and 3-year OS.
In a French phase 3 study (SPIRIT trial), 636 newly diagnosed patients with CML-CP were randomly assigned to receive imatinib (400 mg daily) alone, imatinib (400 mg) plus cytarabine or pegylated interferon (peginterferon) α-2a (90 μg weekly), or imatinib 600 mg daily alone [30]. The primary end points were EFS, PFS, and OS. At 12 months, the rate of molecular response was significantly higher in the imatinib + peginterferon α-2a group than that in imatinib 400 mg alone group (30% vs. 14%; P = 0.001). However, the combination was associated with substantial toxic effect, because of which peginterferon α was discontinued in 46% of the patients.
The Nordic randomized phase 2 study compared imatinib (400 mg daily) monotherapy with imatinib plus peginterferon α-2b (50 μg weekly) after imatinib induction in patients with CML-CP with low and intermediate Sokal risk [31]. The primary end point of MMR rate at 12 months was significantly higher in the former group, when compared with imatinib-alone group (82% vs. 54%, P = 0.002). Peginterferon α-2b was discontinued in 61% of patients in the combination arm. Therefore, the combination of peginterferon α with imatinib, despite the increase in associated toxicity, seems to enhance deep molecular response rates and should offer more possibilities for TKI therapy. These results suggested that addition of peginterferon-α added value to imatinib treatment but were not associated with superior PFS or OS.
3.7 Pharmacokinetics of Imatinib Therapy
Oral bioavailability of imatinib is 98%, and its half-life is approximately 20 h. Therefore, imatinib is administered orally in a once daily regime in patients with CML. Imatinib is metabolized mainly by CYP3A4 isoenzyme. The pharmacokinetics of imatinib tends to show considerable interpatient variability [32]. The mean plasma trough level of imatinib at day 28 was slightly higher in females than that in males (1078 ± 515 ng/mL vs. 921 ± 531 ng/mL). A weak correlation of imatinib trough levels with body weight, body surface area, and the age of patients was observable. However, Larson et al. suggested that effects of these factors on imatinib trough levels are not likely to be clinically significant due to the large interpatient variability in plasma trough concentrations.
The imatinib trough levels were significantly higher in patients who achieved CCyR than those who did not (mean, 1009 ng/mL vs. 812 ng/mL, P = 0.01). Thus, an imatinib trough level of ˃1000 ng/mL appears to be important for achievement of CcyR. Picard et al., too, reported a threshold of 1002 ng/mL to achieve MMR [33].
3.8 Prognostic Factors
Predictive variables for risk of progression and probability of drug discontinuation are required for patients with CML. Three prognostic classifications are currently available, and two of these (the Sokal score and the Euro [Hasford] score) have been used in studies of TKIs [34, 35]. However, the Sokal score was developed for patients treated with conventional chemotherapy, while the Euro score was based on IFNα therapy. In the European Treatment and Outcome Study for CML (EUTOS), a new prognostic risk score based on a study of 2060 imatinib-treated patients was developed to predict the probability of achieving CCyR within 18 months (Table 3.3) [36]. The EUTOS score relies on the percentage of basophils and spleen size to differentiate high-risk from low-risk patients. Other variables (age, platelet count, blast cells, and eosinophils) used in the previous classifications were later shown not to affect the response to imatinib. The EUTOS score predicted that 34% of high-risk patients would fail to achieve a CCyR in 18 months and also predicted a significant difference in PFS (82% for high-risk vs. 90% for low-risk patients, P = 0.006).
Table 3.3
Risk scores for CML-CP
Risk score | Calculation | Risk definition by calculation |
|---|---|---|
Sokal score [34] | Exp 0.0116 × (age − 43.4) + 0.0345 × (spleen – 7.51) + 0.188 × [(platelet count/700)2 – 0.563] + 0.0887 × (blast cells – 2.10) | Low risk: < 0.8 |
Intermediate risk: 0.8–1.2 | ||
High risk: >1.2 | ||
Euro (Hasford) score [35] | 0.666 when age = > 50 year + (0.042 × spleen) + 1.0956 when platelet count > 1500 × 109/L + (0.0584 × blast cells) + 0.20399 when basophils > 3 % + (0.0413 × eosinophils) × 100 | Low risk: = <780 |
Intermediate risk: 781–1480 | ||
High risk: >1480 | ||
EUTOS score [36] | (7 x basophils) + (4 x spleen) | Low risk: = <87 |
High risk: >87 |
In the German CML Study IV, the relationship between comorbidities at diagnosis and overall prognosis were assessed using the Charlson Comorbidity Index (CCI) [37]. Age was associated with a higher CCI score in 863 patients, while comorbidities had an impact on OS. However, comorbidities had no negative effect on response rates and progression to advanced phases. Therefore, OS may not be appropriate for assessment of outcome measures for TKI therapy in CML-CP.
As biological variables, translocation type (standard and variant), additional chromosomal alteration (del der 9, trisomy 8, isochromosome 17, additional loss of material from 22q, and double Ph), transcript type, and baseline BCR-ABL1 kinase domain mutations have been reported. Regarding the leukemic stem cell burden, Mustjoki et al. reported a correlation between the proportion of Ph-positive cells in CD34+ CD38− fraction at diagnosis and the cytogenetic and molecular response [38].
Novel biological prognostic biomarkers for clonal evolution of CML have been identified by use of gene expression profiles, whole exome sequencing, and protein-level profiling.
Radich et al. identified 3000 genes that showed a significant association with the disease phase on DNA microarray analysis [39]. Their findings suggest the progression of CP CML to advanced phase CML as a two-step process. Patients with gene expression patterns of advanced disease may benefit from more aggressive therapies. Recently, targeted deep sequencing has been performed in Ph-positive clones, and mutations of ASXL1, DNMT3A, RUNX1, and TET2 have been identified [40]. These BCR-ABL1-independent gene mutations in patients with CML may be important cofactors in the clonal evolution of CML.
3.9 Monitoring the Response to Treatment of TKIs
Monitoring the response to imatinib requires absolute and differential blood counts, cytogenetic tests, and molecular testing for BCR-ABL1 transcript level and for ABL1 tyrosine kinase domain mutations [7]. According to ELN recommendations for imatinib therapy, blood counts and differentials are required frequently during the first 3 months until CHR has been confirmed. Cytogenetic testing is required at 3 and 6 months, every 6 months thereafter until confirmation of CCyR, and every 12 months thereafter. Real-time quantitative polymerase chain reaction (RQ-PCR) for assessment of BCR-ABL1 transcript levels is recommended every 3 months until MMR has been achieved and confirmed and, at least, every 6 months thereafter (Table 3.4).
Table 3.4
Evaluation of response to first-line TKIs at each time point (months) in ELN recommendations 2013 and the NCCN guidelines for CML version 2.2017
Months | Optimal
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |
|---|---|---|