Organization
Screening recommendations
AAFP
Every 1–2 years, ages 50–69; counsel women ages 40–49 about potential risks and benefits of mammography and clinical breast examination
ACOG
Every 1–2 years starting at age 40, yearly after age 50
ACS
Annually after age 40
AMA
Every 1–2 years in women ages 40–49, annually beginning at age 50
CTFPHC
Every 1–2 years, ages 50–59
NIH
Data currently available do not warrant a universal recommendation for mammography for women in their 40s; each woman should decide for herself whether to undergo mammography
USPSTF
Every 1–2 years, ages 50–69
Table 19.2
Recommendations for mammographic screening in women aged 40–49 years
Organizations that recommend routine screening | Organizations that do not recommend routine screening |
|---|---|
American Cancer Society | American Academy of Family Physicians |
American College of Obstetricians and Gynecologists | American College of Physicians |
American College of Radiology | Canadian Task Force on Periodic Health Exams |
American College of Surgeons | National Institutes of Health Consensus Panel |
National Cancer Institute | US Preventive Services Task Force |
Table 19.3
Special recommendations by the American College of Radiology
Special recommendations |
|---|
For BRCA1 or BRCA2 mutation carriers, untested first-degree relatives of BRCA mutation carrier, or a first-degree relative affected young, screening should be started by the age of 30 |
Patients with a personal history of atypical duct hyperplasia or lobular carcinoma in situ could benefit of been performed a mammography every 6 months, even could being candidates for a breast RMI |
History of high-dose chest irradiation received between the ages of 10 and 30 should begin with an annual mammogram study and, recommended, an annual RMI, 8 years after the exposure |
Imaging Techniques for the Diagnosis of Breast Cancer
Mammography
Mammography is the only screening test proven to decrease breast cancer morbidity and mortality and it has been used during the last century. Mammography uses low-dose X-ray; high-contrast, high-resolution film; and an X-ray system designed specifically for imaging the breasts. Although it is convenient to analyze mammography independently of other imaging techniques, in the clinical practice, it is highly recommended to use it in combination with those others, especially ultrasound [23].
In the United States, the incidence of breast cancer is 3 in 1,000 women and the rate of restudy of the mammography screening is 8 %. Seven percent of the women screened will need just another mammography or ultrasound scan and only 1 % will need a biopsy to detect those 0.3 % women with breast cancer [24]. Those referred for additional diagnostic testing and further views may be studied by another mammography or special mammography views, breast ultrasound, or other adjunctive imaging such as a magnetic resonance (MR), digital mammography, sestamibi, or T-scan.
The US Food and Drug Administration (FDA) reports that mammography can find 85–90 % percent of breast cancers in women over 50 and can discover a lump up to 2 years before it can be felt. In 1994, the equipment, quality of operations, technologies, and doctors were regulated by the Mammography Quality Standards Act (MQSA).
A mammogram is like a fingerprint; the appearance of the breast on a mammogram varies tremendously from woman to woman, and no two mammograms are alike. It is extremely helpful for the radiologist to have films (not just the report) available from previous examinations for comparison purposes. This will help the doctor to recognize small changes that occur gradually over time and detect a cancer as early as possible.
A basic knowledge is required for the analysis of the mammography. The typical equipment produces low-dose X-ray (25–30 kVp) while the breast is compressed. The compression of the breast is necessary to limit the radiation dose and to improve the quality of the images. The X-ray is radiated through the compressed breast and onto a film cassette positioned under the breast. The X-rays hit a special phosphor coating inside the cassette. This phosphor glows in proportion to the intensity of the X-ray beams hitting it, thus exposing the film with an image of the internal structures of the breast. As the X-rays pass through the breast, they are attenuated (weakened) by the different tissue densities they encounter. Fat is very dense and absorbs or attenuates a great deal of the X-rays. The connective tissue around the breast ducts and fat is less dense and attenuates or absorbs far less X-ray energy. It is these differences in absorption and the corresponding varying exposure level of the film that create the images which can clearly show normal structures such as fat, fibroglandular tissue, breast ducts, and nipples. Quality of the images can be affected by many factors, particularly high density of the breast tissue, the thickness of the breast compressed, the position, the movement, and the radiation dose.
Currently, there are two kinds of receptors approved by the FDA: film-screen mammography and digital mammography, also called full-field digital mammography, or FFDM. The last one will be explained later in this chapter. The technique for performing both is the same; what differs is whether the images take the form of photographic films or of digital files recorded directly onto a computer.
Mammography Classification
There are two types of mammography exams, screening and diagnostic:
Screening mammography is the X-ray examination of the breasts when the woman is asymptomatic, which means in a preclinical stage. This is the goal of screening mammography and its objective is the higher sensitivity to be able to detect any anomaly. For screening mammography, each breast is imaged separately, typically from above (craniocaudal view or CC) and from an oblique view (mediolateral-oblique or MLO), as shown in Fig. 19.1 [25]. The mediolateral-oblique (MLO) view is probably the most important and most common view taken followed by the craniocaudal view (CC). Figure 19.2 shows a normal screening mammography.
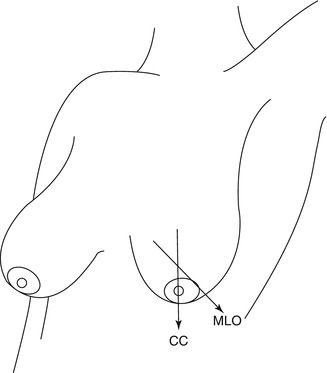
Fig. 19.1
Mammography projections. CC Cranial-caudal view, MLO Mid-Lateral Oblique view
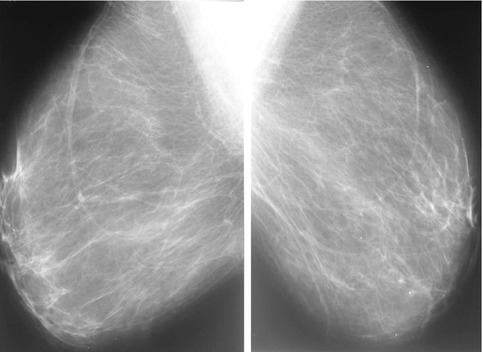
Fig. 19.2
Normal screening mammography
Diagnostic mammography is the X-ray examination of the breasts in a woman who either has a breast complaint (for instance, a breast lump or nipple discharge found during self-exam) or has had an abnormality found during screening mammography. Diagnostic mammography is more involved and time-consuming than screening mammography and is used to determine exact size and location of breast abnormalities and to image the surrounding tissue and lymph nodes. Typically, several additional views of the breast are imaged and interpreted during diagnostic mammography, especially in women with breast implants or a personal history of breast cancer. Thus, diagnostic mammography is more expensive than screening mammography. Figure 19.3 shows an abnormal mammography.
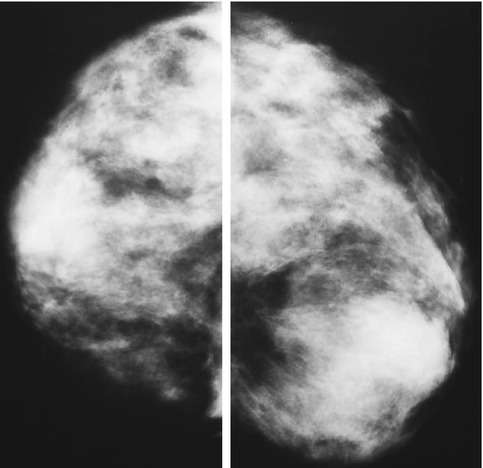
Fig. 19.3
Abnormal findings in mammography
Mammogram Analysis
The first thing to do when a mammogram is analyzed is to detect any anomaly; the next step is its classification. The American College of Radiology (ACR) has established the Breast Imaging Reporting and Database System (BI-RADSTM) [21, 26] to guide the breast cancer diagnostic routine. Each BI-RADS category is often referred as a “level” in radiologists’ terms.
The BI-RADS categories are used to standardize interpretation of mammograms among radiologists. They are useful for statistical analysis of mammography practice, and the results are compiled on a nationwide basis in the United States to help refine mammography procedures everywhere. Table 19.4 shows a summary of the BI-RADS categories.
Table 19.4
BI-RADS mammography categories according to the American College of Radiology
BI-RADS assessment categories | |
|---|---|
Category 0 | Need additional imaging evaluation |
Category 1 | Negative. Keep screening |
Category 2 | Benign finding. Keep screening |
Category 3 | Probably benign finding. Short interval of follow-up is suggested |
Category 4 | Suspicious abnormality. Biopsy should be considered |
Category 5 | Highly suggestive of malignancy. Appropriate action should be taken |
Each BI-RADS level has an appropriate management or follow-up plan associated with it (see below). Furthermore, if used correctly and consistently, each BI-RADS category has the following risk of malignancy and meaning:
Category 0 is a temporary category, which means that additional imaging is needed before assigning a permanent BI-RADS category. Most Category 0 findings are shown to be benign once the image study is completed.
Category 1 means that the screening is negative and the chance of having a breast cancer is 5 in 10,000. That also means the woman can continue with the screening established.
Category 2 means that the images found are benign or non-suspicious of being cancerous. It also indicates the same plan of follow-up as that of Category 1. This is the typical category where cases of cysts or fibroadenomas are classified.
Category 3 means that the image found is probably benign, but there is still less than a 2 % chance of cancer. It also means that another mammography is recommended in 6 months. However, most of the findings classified as Category 3 abnormalities do not receive a biopsy.
Category 4 means that the abnormality is suspicious of malignancy. Although most of the images classified as Category 4 are found to finally be benign, they require a biopsy since they have a 25–50 % rate of malignancy.
Category 5 means that some classic signs of malignancy are seen in the mammography, which are highly suggestive of cancer. It also means that all Category 5 abnormalities typically receive biopsy. If the biopsy results are benign, the abnormality usually receives re-biopsy since the first biopsy may not have sampled the correct area. The percentage of Category 5 abnormalities that will be cancer may vary between 75 and 99 %.
Findings Descriptions
When viewing a mammogram, it is important to know the exact orientation of the image. The breasts are best viewed as symmetric organs. Comparison of the right breast to the left breast is done for evaluation of symmetry. Perceptual psychologists have shown that the eye can more easily perceive asymmetric densities when patterns are scanned in a mirror-image fashion rather than side by side. Therefore, the conventional method is to evaluate mammograms in a mirrorlike fashion with both the MLO and CC views mounted back to back.
What is normally identified in mammograms are masses, calcifications, areas of asymmetric density, and areas of distortion of the normal breast architecture [27]. Most of the breast cancers visible in mammographies were detected as a mass, calcifications, distortion of architecture, or a combination of these [28–32].
Masses. Masses are three-dimensional lesions which may represent a localizing sign of breast cancer. They are described by their localization, size, shape (round, oval, lobulated, irregular or architectural distortion), margins (circumscribed, obscured, microlobulated, non-defined, spiculated), X-ray attenuation, effect in surrounding tissue, and other associated findings. Depending on the morphological criteria of the mass, the likelihood of malignancy can be established.
Calcifications. Calcifications are often important and common findings on a mammogram. They can be produced from cell secretion or from necrotic cellular debris. They may be intramammary in many different locations but, alternatively, they may be found in the skin. They can appear with or without an associated lesion, and their morphology and distribution provide clues as to their etiology and its association with a benign or malignant process. Calcifications found within or around a mass provide further information about that particular mass. For example, an involuting fibroadenoma will often contain “popcorn-like” macrocalcifications. Similarly, fine curvilinear calcifications at the margin of a round or oval mass indicate a benign process. On the other hand, a mass with pleomorphic, irregularly shaped calicifications, which is also heterogeneous in size and morphology, raises much greater concern about malignancy. Calcifications are analyzed according to their size, shape, number, and distribution. The general rule is that larger, round, or oval-shaped calcifications uniform in size have a higher probability of being associated with a benign process and smaller, irregular, polymorphic, branching calcifications heterogeneous in size and morphology are more often associated with a malignant process. Certain calcification patterns are almost always pathognomic of a benign process, and in such cases, no further analysis is needed. In the majority of cases, however, a pattern of calcification deposition is inconclusive and may be attributable to either a benign or malignant process. The BI-RADS system has also classified findings of calcifications into three categories: (1) typically benign, (2) intermediate concern, and (3) higher probability of malignancy.
Areas of distortion of asymmetric density. The breasts are seen as symmetric structures and should be compared as such. Although exact mirror images cannot be expected, patterns within each breast should be similarly distributed. An asymmetric area may indicate a developing mass, a variation of the normal breast tissue, any postoperative change from a previous biopsy or surgery, or merely poor positioning and compression during imaging. The appearance of asymmetries due to positioning and compression during imaging is often the result of superimposition of normal breast structures. Nevertheless, true breast asymmetry is three-dimensional and should be present on both MLO and CC views. Once an asymmetry is determined to be three-dimensionally real, the interpreter must determine whether the asymmetry is a benign variation of asymmetric breast tissue or a focal asymmetric density that may represent a significant mass.
Areas of distortion of the normal breast. There is no one breast pattern that may be classified as “normal.” Only from experience can one appreciate the variations of a normal-looking mammogram. Getting familiar with the spectrum of a “normal” appearance is to a certain extent essential to detecting abnormalities.
Common Findings
Fibroadenoma. It is the most common benign, solid growth in the breasts, especially in young women. It develops under the influence of estrogen. Its mammographic appearance is a mass with sharply well-demarcated margin, and it is virtually indistinguishable from a cyst or a well-circumscribed carcinoma. For this reason, it is impossible to identify a fibroadenoma radiographically without additional mammographic features. The additional features that allow one to distinguish a fibroadenoma have to do with the fact that fibroadenomas often regress with menopause. During regression, the noncalcified appearance changes and calcifications develop. The typical involuting fibroadenoma contains popcorn-like macrocalcifications and is easily identified on a mammogram. Fibroadenoma has no significant risk of becoming cancer and does not put a patient at increased risk of breast cancer.
Cysts. They are harmless accumulations of fluid in the breast (and we can see them in other tissues or organs). As noted, a noncalcified fibroadenoma is indistinguishable from a cyst radiographically. That is, cysts generally have clearly defined margins radiographically when not obscured by surrounding tissue parenchyma. Cysts occur as a result of the dilatation of the lactiferous ducts within the lobules due to the imbalance between secretion and resorption. The exact causes of cysts are not known, but cysts are known to change with hormonal variations, either during normal menstrual cycles or from postmenopausal hormone replacement therapy. They commonly occur in women between 30 and 50 years of age. Cysts do not become cancer or increase the risk of cancer. Most of the time, cysts may be left alone, but sometimes a physician may drain them with a small needle (fine-needle aspiration).
Abscess. It is a benign lesion which may or may not appear round and well circumscribed. Sometimes abscesses are associated with acute mastitis and are often resolved clinically. Lacking a clinical history, only needle aspiration can diagnose them.
Intraductal papillomas. They result from a proliferation of ductal epithelial tissue. They are frequently too small to be evident on a mammogram, but if they grow large enough, they can appear as circumscribed masses and, in certain cases, may even obstruct the duct and give it the appearance of duct dilatation.
Intramammary lymph node. Normal lymph nodes are usually small, without calcifications, and have well-defined margins and shape. In the oblique mammography view, they are normally found in the axilla. On the other hand, intramammary lymph nodes are sometimes interpreted as suspicious breast masses. Magnification may be helpful to identify additional mammographic features of a hilus and central fat, in which case the likelihood of it being benign is increased.
Factors That May Affect Sensitivity and Specificity of Mammography
How mammograms can detect malignant lesions may vary in every woman. The most important factors that can modify sensitivity and specificity are the age of the patient, the density of the breast, having hormonal substitutive treatment, and the different types of breast cancer. A critical factor determining mammographic sensitivity and specificity is also the radiologist’s interpretation. It is important not to forget these factors:
Breast density. There is enormous variability in density among breasts, from those that are almost entirely fibroglandular in appearance to those that are almost entirely fatty in appearance. High breast density is associated with low sensitivity [33, 34]. Breast cancer attenuates X-rays and appears as a white density. A white density against a black (fatty) background is easy to detect (high signal-to-noise ratio). A white density cancer against a white background of fibroglandular tissue is difficult and, in many situations, impossible to detect. The normal dense tissue camouflages the cancer. Extensive breast density has been associated with higher frequency of false-negative mammograms. At all ages, regardless of hormone therapy (HT), high breast density is associated with 10–29 % lower sensitivity [35]. The relative insensitivity of mammography in women with dense breasts is a significant limitation of the technique.
Age. Breast density generally declines with age. Therefore, excluding cases where patients were HT users, sensitivity above 65 years old is better than in younger women, not only due to this lower breast density but also due to the fibrocystic changes, and even the growing rates are lower [36, 37].
Hormonal therapy treatment. HT increases breast density. That fact brings up two problems when mammograms are analyzed. The first is due to the high density itself, which may camouflage suspicious lesions (see above); but the second concern is that the radiologist may not be able to know if that increment in breast density is due to the HT treatment or to a malignant lesion in progress [38].
Biological subtypes of breast cancer. Invasive lobular carcinoma may be difficult to detect in early stages [39, 40], not only due to its characteristic growth pattern but also because it is only associated with calcifications in just 5 % of the cases.
Radiologist’s interpretation. There are many studies published showing substantial variability in interpretation and reading accuracy among radiologists. The clinical significance of variability in radiologists’ interpretations is not clear [41]. Identifying a radiologist who is more accurate than another is difficult.
Technical factors. There are many technical factors that may decrease sensitivity and specificity of the mammography. To start with, some areas of the breast are sometimes hidden in mammograms. The quality of the image is also reduced in thicker breasts due to a significant loss of contrast. Another example is breast prostheses that, when located in front of the muscle, can camouflage many lesions.
On the other hand, international comparisons of screening mammography have found that specificity is greater in countries with more highly centralized screening systems and national quality assurance programs. For example, one study reported that the recall rate is twice as high in the United States as it is in the United Kingdom, with no difference in the rate of cancers detected [42]. Such comparisons may be confounded, however, by other social, cultural, or economic factors that can influence the performance of mammography screening. No improvement in cancer detection was noted in these studies, despite the higher recall rate.
Breast Ultrasound
As a breast cancer detection procedure, ultrasound cannot replace a mammogram for breast cancer screening. Screening breast ultrasound as a possible replacement for mammography was tried unsuccessfully in the early 1970s. Supported by expert opinion, the European Group for Breast Cancer Screening concluded that there is little evidence to support the use of ultrasound in population breast cancer screening at any age [43].
Following this failed attempt, breast ultrasound fell into some degree of disrepute. In overreaction, many breast imagers in the United States loss confidence in breast ultrasound for most of a decade for any purpose other than distinguishing cyst from solid. Gradually, through the 1990s and 2000s, breast ultrasound has reemerged as the key and first diagnostic breast modality that is used after mammography. Its diagnostic and guidance roles continue to expand and evolve, and recently we have begun the process of reevaluating ultrasound as an ancillary screening tool that is used after mammography in women with high risk, dense breast tissue, or both. Moreover, it is the election technique in women who cannot be exposed to X-ray (women under 30 years or pregnant women) and is also very useful in women with breast implants because breast ultrasound is more sensitive for the evaluation of extracapsular and intracapsular rupture than mammography [44–47].
Over the past two decades, one of the advances in medicine and imaging research has been the marked expansion of the capabilities of breast ultrasound in the evaluation of breast disease. Breast ultrasound has become a fundamental component for the diagnosis and prognosis of breast cancer. However, breast ultrasound also has some drawbacks, which include its relatively higher cost compared with screening mammography, operator-skill dependence, difficulty in providing reproducible results between different facilities, and the time required to carry out the study. Perhaps the biggest shortcoming of ultrasound is its higher false-negative rate, when compared with mammography, for general screening, especially for the malignant microcalcifications that are typically better seen mammographically [48]. We may see some limitations of breast ultrasounds, as noted here:
Breast Ultrasound Limitations
Deep location of the lesion in the breast
Cannot detect most calcifications in breast lesions
The ultrasound contrast between the lesion and the surrounding breast tissue
Experience and qualification of operator
Quality of the equipment used
Difficulty in reevaluation of images after the exam
A key to understanding ultrasound is knowledge of the nature of the ultrasound transducer. A transducer is, fundamentally, a device that converts one form of energy to another. Modern ultrasound transducers are handheld units that convert electric signals into ultrasonic energy that is then transmitted into the tissues. Typically, a piezoelectric crystal near the face of the transducer generates high-frequency sound when voltage is applied. The sonic beams used in diagnostic breast ultrasound typically have frequencies of more than 7 million cycles per second (7 MHz). Following interaction of the sound waves with the tissues, the transducer receives and reconverts ultrasound energy back into an electric signal, which is used to create the image.
The use of high-resolution breast ultrasound equipment is important, and a dedicated breast ultrasound unit is preferable. High-frequency linear array transducers are required because linear transducers have a wider near field and can more easily guide intervention procedures. The 2000–2001 American College of Radiology (ACR) Standard for the Performance of Breast Ultrasound Examination suggests transducer frequencies of 7 MHz or higher. If broadband systems are used, the ACR standard states that a center frequency of 6 MHz or higher is needed. Current transducer frequencies are typically 10 MHz or higher. If possible, color Doppler capability should also be available, as explained below.
A transducer of the correct frequency should be used so that the frequency is appropriate to the size and depth of the area of abnormality. The ACR standard states that the frequency should be high enough to permit differentiation of fluid versus solid breast masses; however, that is not always possible.
Placing the patient in a supine position minimizes the depth of tissue penetration needed for imaging by the ultrasound beam [49]. Raising the ipsilateral hand behind the head flattens the breast and minimizes the tissue depth. For lateral lesions, the ACR standard suggests supine-oblique positioning for scanning. Turning the patient away from the side to be examined flattens the lateral tissue against the chest wall. For medial lesions, the supine position is preferred and it is highly recommended to palpate the lesion while scanning. Skin and superficial breast tissue lesions are better visualized with higher frequency transducers. Scanning of the retroareolar region is often limited by shadowing from the nipple, but angling the transducer behind and beneath the nipple helps to solve this problem.
The interpreting physician should be able to understand triangulation principles for mammographic abnormalities and to correlate breast ultrasound with mammograms [50].
Breast Ultrasound Indications
There are many different uses for a breast ultrasound, including:
Palpable abnormalities (Fig. 19.4)
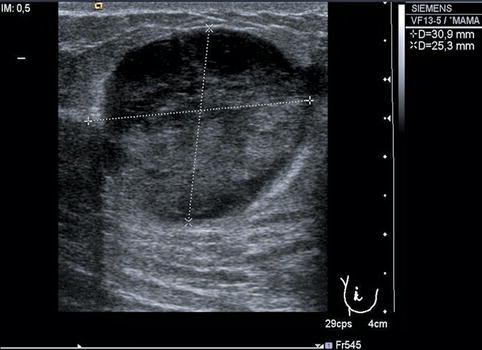
Fig. 19.4
Breast ultrasound scan evidencing a tumoral mass
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree