Imaging plays an important role in the management of patients with testicular germ cell tumors. This article reviews the role of imaging in the diagnosis, staging, and posttreatment restaging of these patients. CT remains the main radiologic technique used, although ultrasound, MRI, and positron emission tomography also have specific roles in the clinical management of these patients.
In the management of patients with testicular germ cell tumors (TGCT), imaging plays a pivotal role. Although sonography of the testes is useful for the identification of a testicular mass, the definitive diagnosis of a testicular tumor is usually reliant on a biopsy or orchidectomy. Once the diagnosis of a testicular neoplasm is established, imaging is crucial for defining the presence and extent of metastatic disease, assessing disease response to treatment, evaluating the suitability of residual masses after chemotherapy for surgery, and detecting sites of relapse. Computed tomography (CT) remains the main radiologic technique for disease evaluation. However, other imaging techniques, such as chest radiography, MRI, positron emission tomography using 18-fluoro-2-deoxyglucose (FDG-PET), and ultrasound all have specific roles in the clinical management of these patients.
Diagnosis
The diagnosis of GCT tumors is usually made on biopsy or at orchidectomy. TGCT most commonly presents as a painless palpable mass but occasionally may present with nonspecific symptoms, such as dull scrotal ache, pain, or acute fever. In patients with retroperitoneal metastases or disseminated disease, backache, malaise, lethargy, gynecomastia, and other systemic features may be the presenting symptoms.
Testicular ultrasound (which should be performed using a high-resolution 7.5-MHz probe) is used for the primary assessment of the testes to confirm the presence of a testicular mass ( Fig. 1 ); to distinguish these from other scrotal abnormalities; and to screen for associated abnormalities, such as microlithiasis. Testicular ultrasound is also helpful for assessment in young male patients who present with metastatic disease, in whom an occult primary tumor of the testis is suspected. Ultrasound is also used to examine the contralateral testis of a patient confirmed to have testicular tumor, to identify the small number of patients who may present with bilateral synchronous tumors.
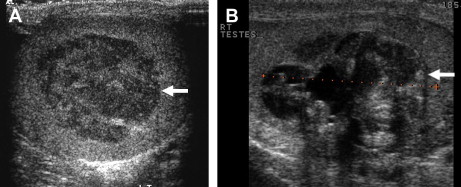
Sonographically, testicular tumors are usually well-defined and hypoechoic relative to the normal testicle, although some may display heterogeneous echo-texture, calcification, or cystic change. Tumors may display increased vascularity on color and power Doppler with respect to surrounding normal testicular tissue but this is not specific and may not be demonstrable in small tumors. Ultrasound cannot reliably differentiate between tumor types, and for this purpose MRI may be useful.
MRI has been reported to be able to distinguish between seminoma and nonseminomatous GCT (NSGCT). However, this may be of little practical value because appropriate management dictates orchidectomy to obtain detailed histopathology of the tumor, which is mandatory for primary treatment. Nevertheless, MRI of the scrotum may be useful if clinical and sonographic assessment cannot differentiate between an intratesticular or extratesticular mass. A further role for MRI of the scrotum is the preoperative evaluation of the local extent of malignant testicular tumors in patients for whom testis-sparing surgery is planned, such as those with bilateral GCTs or tumor in a solitary testis.
Staging
Before initiating therapy, assessment of disease extent must be performed. Guidelines from the National Comprehensive Cancer Network and the European Germ Cell Cancer Consensus Group recommend that TNM staging be used ( Table 1 ) and that patients with metastatic disease are further categorized using the International Germ Cell Cancer Collaborative Group classification, which stratifies patients into good, intermediate, and poor prognostic groups. This latter classification is based on histology, location of primary tumor and metastases, and levels of serum markers ( Box 1 ). These guidelines also state that patients should have initial staging with a chest radiograph and CT of the abdomen and pelvis. A CT of the chest is performed if the chest radiograph is abnormal or abdominopelvic CT shows metastatic disease.
| Primary Tumor | |
|---|---|
| The extent of the primary tumor is classified after radical orchidectomy (pT) | |
| pTX | Primary tumor cannot be assessed (if no radical orchidectomy has been performed, TX is used) |
| pT0 | No evidence of primary tumor (eg, histologic scar in testis) |
| pTis | Intratubular germ cell neoplasia |
| pT1 | Tumor limited to testis and epididymis without vascular/lymphatic invasion; tumor may invade into the tunica albuginea but not tunica vaginalis |
| pT2 | Tumor limited to testis and epididymus with vascular/lymphatic invasion, or tumor extending through tunica albuginea with involvement of tunica vaginalis |
| pT3 | Tumor invades spermatic cord with or without vascular/lymphatic invasion |
| pT4 | Tumor invades scrotum with or without vascular/lymphatic invasion |
| Regional Lymph Nodes | |
| Clinical Involvement | |
| NX | Regional nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension or multiple lymph nodes none >2 cm in greatest dimension |
| N2 | Metastasis with a lymph node mass >2 cm but <5 cm in greatest dimension, or multiple lymph nodes, any one mass >2 cm but ≤5 cm in greatest dimension |
| N3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| Pathologic involvement | |
| pN0 | No regional lymph node metastases |
| pN1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension and 5 or fewer positive nodes, none >2 cm in greatest dimension |
| pN2 | Metastasis with a lymph node mass >2 cm but ≤5 cm in greatest dimensions; or more than five nodes positive, none >5 cm; or evidence of extranodal extension of tumor |
| pN3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| Distant Metastases | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Nonregional lymph node or pulmonary metastasis |
| M1b | Distant metastasis other than to nonregional lymph nodes and lungs |
| Serum tumor markers (S) | |
| SX | Tumor marker studies not available or not performed |
| S0 | Tumor marker levels within normal limits |
| S1 | LDH <1.5 normal and HCG (mIu/ml) <5000 and AFP (g/ml) <1000 |
| S2 | LDH 1.5–10 normal or HCG (mIu/ml) 5000–50,000 or AFP (g/ml) 1000–10,000 |
| S3 | LDH >10 normal or HCG (mIu/ml) >50,000 or AFP (g/ml) >10,000 |
| Stage Groupings | |
| • Stage I | pT1-4, N0, M0, SX |
| • Stage II | Any pT/Tx, N1-3, M0, SX |
| ○ Stage IIA | Any pT/Tx, N1, M0, S0 Any pT/Tx, N1, M0, S1 |
| ○ Stage IIB | Any pT/Tx, N2, M0, S0 Any pT/Tx, N2, M0, S1 |
| ○ Stage IIC | Any pT/Tx, N3, M0, S0 Any pT/Tx, N3, M0, S1 |
| • Stage III | Any pT/Tx, Any N, M1, SX |
| ○ Stage IIIA | Any pT/Tx, Any N, M1a, S0 Any pT/Tx, Any N, M1a, S1 |
| ○ Stage IIIB | Any pT/Tx, N1-3, M0, S2 Any pT/Tx, Any N, M1a, S2 |
| ○ Stage IIIC | Any pT/Tx, N1-3, M0, S3 Any pT/Tx, Any N, M1a, S3 Any pT/Tx, Any N, M1b, Any S |
- •
Nonseminoma
- ○
Good prognosis: all of the following
- ▪
AFP <1000 ng/mL and HCG <5000 IU/L (1000 ng/mL) and LDH <1.5 upper limit of normal (N) and
- ▪
Nonmediastinal primary
- ▪
No nonpulmonary visceral metastases (NPVM)
- ▪
- ○
Intermediate prognosis: all of the following
- ▪
AFP 1000–10,000 ng/mL, or HCG 5000–50,000 IU/L, or LDH 1.5–10 N and
- ▪
Nonmediastinal primary site and
- ▪
No NPVM
- ▪
- ○
Poor prognosis: any of the following
- ▪
AFP >10,000 ng/mL or HCG >50,000 IU/L or LDH >10 N or
- ▪
Mediastinal primary site or
- ▪
NPVM
- ▪
- ○
- •
Seminoma
- ○
Good prognosis
- ▪
No NPVM
- ▪
Any primary site
- ▪
Normal AFP, any HCG, any LDH
- ▪
- ○
Intermediate prognosis
- ▪
NPVM present
- ▪
- ○
Abbreviations: AFP, -fetoprotein; B-HCG, B-human gonadotrophin; CNS, central nervous system; LDH, lactate dehydrogenase.
Staging
Before initiating therapy, assessment of disease extent must be performed. Guidelines from the National Comprehensive Cancer Network and the European Germ Cell Cancer Consensus Group recommend that TNM staging be used ( Table 1 ) and that patients with metastatic disease are further categorized using the International Germ Cell Cancer Collaborative Group classification, which stratifies patients into good, intermediate, and poor prognostic groups. This latter classification is based on histology, location of primary tumor and metastases, and levels of serum markers ( Box 1 ). These guidelines also state that patients should have initial staging with a chest radiograph and CT of the abdomen and pelvis. A CT of the chest is performed if the chest radiograph is abnormal or abdominopelvic CT shows metastatic disease.
| Primary Tumor | |
|---|---|
| The extent of the primary tumor is classified after radical orchidectomy (pT) | |
| pTX | Primary tumor cannot be assessed (if no radical orchidectomy has been performed, TX is used) |
| pT0 | No evidence of primary tumor (eg, histologic scar in testis) |
| pTis | Intratubular germ cell neoplasia |
| pT1 | Tumor limited to testis and epididymis without vascular/lymphatic invasion; tumor may invade into the tunica albuginea but not tunica vaginalis |
| pT2 | Tumor limited to testis and epididymus with vascular/lymphatic invasion, or tumor extending through tunica albuginea with involvement of tunica vaginalis |
| pT3 | Tumor invades spermatic cord with or without vascular/lymphatic invasion |
| pT4 | Tumor invades scrotum with or without vascular/lymphatic invasion |
| Regional Lymph Nodes | |
| Clinical Involvement | |
| NX | Regional nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension or multiple lymph nodes none >2 cm in greatest dimension |
| N2 | Metastasis with a lymph node mass >2 cm but <5 cm in greatest dimension, or multiple lymph nodes, any one mass >2 cm but ≤5 cm in greatest dimension |
| N3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| Pathologic involvement | |
| pN0 | No regional lymph node metastases |
| pN1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension and 5 or fewer positive nodes, none >2 cm in greatest dimension |
| pN2 | Metastasis with a lymph node mass >2 cm but ≤5 cm in greatest dimensions; or more than five nodes positive, none >5 cm; or evidence of extranodal extension of tumor |
| pN3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| Distant Metastases | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Nonregional lymph node or pulmonary metastasis |
| M1b | Distant metastasis other than to nonregional lymph nodes and lungs |
| Serum tumor markers (S) | |
| SX | Tumor marker studies not available or not performed |
| S0 | Tumor marker levels within normal limits |
| S1 | LDH <1.5 normal and HCG (mIu/ml) <5000 and AFP (g/ml) <1000 |
| S2 | LDH 1.5–10 normal or HCG (mIu/ml) 5000–50,000 or AFP (g/ml) 1000–10,000 |
| S3 | LDH >10 normal or HCG (mIu/ml) >50,000 or AFP (g/ml) >10,000 |
| Stage Groupings | |
| • Stage I | pT1-4, N0, M0, SX |
| • Stage II | Any pT/Tx, N1-3, M0, SX |
| ○ Stage IIA | Any pT/Tx, N1, M0, S0 Any pT/Tx, N1, M0, S1 |
| ○ Stage IIB | Any pT/Tx, N2, M0, S0 Any pT/Tx, N2, M0, S1 |
| ○ Stage IIC | Any pT/Tx, N3, M0, S0 Any pT/Tx, N3, M0, S1 |
| • Stage III | Any pT/Tx, Any N, M1, SX |
| ○ Stage IIIA | Any pT/Tx, Any N, M1a, S0 Any pT/Tx, Any N, M1a, S1 |
| ○ Stage IIIB | Any pT/Tx, N1-3, M0, S2 Any pT/Tx, Any N, M1a, S2 |
| ○ Stage IIIC | Any pT/Tx, N1-3, M0, S3 Any pT/Tx, Any N, M1a, S3 Any pT/Tx, Any N, M1b, Any S |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree