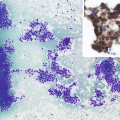
Fig. 19.1
A 40-year-old female with papillary thyroid carcinoma and positive nodal metastases on dissection. TBI (a) with SPECT (b) showing uptake in the neck, which localizes to the thyroid bed. No new metastases were found
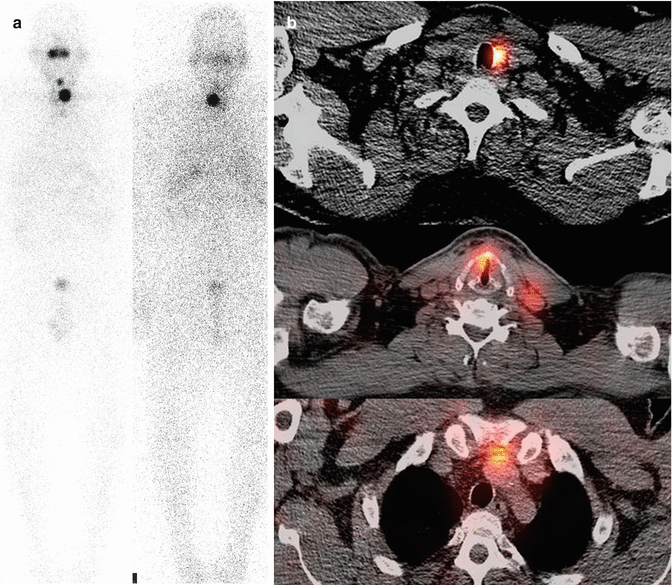
Fig. 19.2
A 60-year-old man with postablation scan for follicular carcinoma localized to the thyroid. TBI (a) with SPECT (b) shows spread to local nodes in the neck and chest
After ablating with radioactive iodine, patients are usually followed with thyroglobulin levels to detect recurrence. For routine follow-up, ultrasound is recommended at least once and possibly more often, whereas TBI is usually only performed once as long as the scan is negative—and that only if the patient is high risk. There is no role for CT or MR in routine follow-up of these patients.
Should serum thyroglobulin rise, imaging may be indicated to evaluate recurrent disease. TBI is usually performed first to localize any iodine-avid lesions. If there is elevated thyroglobulin but TBI scan is negative, FDG-PET/CT may be useful to detect non-iodine-avid disease. TBI and FDG-PET imaging are felt to be complimentary, with radioiodine imaging more sensitive for well-differentiated disease and FDG imaging more sensitive for detecting tumors after they dedifferentiate.
US, CT, and MRI are useful if there is clinically palpable disease or concern for invasion into surrounding neck structures.
Specific Modalities in Imaging
Ultrasound
Primary Role: Local Metastasis and Recurrence
Ultrasound has a prominent role in the initial workup of the primary tumor, in forming a picture of the thyroid and detecting suspicious nodules, and in guiding FNA (fine-needle aspiration) of suspicious nodules. It can also detect nodal recurrence in the neck. Ultrasound scanning is convenient and less expensive compared to CT, MRI, or PET and does not involve ionizing radiation. However, ultrasound imaging is highly operator dependent, and the skill and experience of the technologist and interpreting physician play an important role.
Preoperative Evaluation
Ultrasound examination is recommended for detecting the presence of suspicious cervical nodes in the central or lateral compartments for all patients being investigated for a thyroid nodule or being prepared for thyroidectomy [2], as well as for all patients with cytologic findings positive for malignancy [3] (Table 19.1). Ultrasound is not effective for evaluating distant metastases from thyroid cancer, which may involve the lung and bone.
Table 19.1
Applications by modality
Modality | Applications | |
|---|---|---|
Ultrasound | Follow-up at 6–12 months and “periodically” thereafter | |
Evaluation of palpable or symptomatic abnormalities | ||
Guides biopsy if nodes over 8–10 mm | ||
CT/MR | Neck and upper chest | Bulky and widely distributed nodal disease not well seen by ultrasound |
Investigation of entire aerodigestive tract for invasion | ||
Rising thyroglobulin with negative US | ||
CT chest | High-risk DTC with Tg > 10 ng/mL or rising antibodies | |
CT/MR abdomen | High-risk DTC with Tg > 10 ng/mL and negative neck and chest imaging | |
MR skeletal survey | ||
MR brain | Concern for tumor swelling with radioiodine ablation therapy | |
TBI +/− SPECT | Follow-up at 6–12 months for intermediate- to high-risk patients (SPECT if positive finding) | |
FDG-PET/CT | High-risk patients with Tg > 10 ng/mL and negative TBI | |
Nodal metastases can be identified in 25–50% of patients depending on the technique [4–6]. Micrometastases may be present in a greater number of patients, 53–90% according to some studies [6, 7], although these are of questionable clinical significance—subclinical nodes have a much lower probability of recurrence [8]. Ultrasound may detect about 37% of nodes [9] in older studies, identifying adenopathy in 24–27% of patients [9, 10] and changing management in 20–40% [11–13]. A meta-analysis of 13 studies performed through 2010 gave an overall sensitivity of 72% with a specificity of 98%; sensitivity was better in the lateral compartment than in the central on a per-lesion basis (72% vs. 45%, with overall per-lesion sensitivity of 63%) [14]. More recent work yields similar results, with three studies of at least 100 patients giving 23–41% sensitivity in the central compartment [15–17]. Sensitivity in the lateral compartment (presumably not blocked by the thyroid gland) is again better, at 70–78% [15, 17], although it may be somewhat lower at level V [18] and seems to vary with operator experience [19].
When nodes are found, ultrasound can also be used to suggest the probability of malignancy of a lymph node. Typically, a benign node is oval with a hyperechoic stripe and has vascular flow in the center of the node [3]. Conversely, a round shape, microcalcifications, a cystic appearance, and peripheral vascularity suggest malignancy. Nodes over 1 cm are more likely to be malignant. These features differ in their importance, with features such as microcalcifications and cystic aspect being more specific and features such as peripheral vascularity and hyperechogenicity and the absence of an echogenic hilum being more sensitive [20, 21]. Ultrasound-guided FNA of sonographically suspicious nodes of at least 8–10 mm in short axis is recommended [2]. In addition, patients with significant, posterior or inferiorly located, or poorly visualized adenopathy, fixation to surrounding structures of nodes, or suspicion for significant extrathyroidal extension may benefit from cross-sectional imaging [3].
Unfortunately, ultrasound can also detect many smaller nodes of uncertain significance, and it is not clear which ones will grow (or whether growth indicates morbidity). A survey of 166 patients with suspicious nodes of an average size of 1.3 cm outside the thyroid bed followed over 3.5 years found that only 20% grew, and none cause morbidity or mortality; unfortunately, no sonographic feature predicted growth [22]. Pathological studies give rates of nodal metastasis of 70–90% to the central compartment [23] and 64% in the lateral [24]; however, regional recurrence rates are much smaller, and these nodes may not affect overall, disease-free, or disease-specific survival [23]. At least one study of 560 patients showed that ultrasound-detectable metastases to the lateral compartment predicted a worse relapse-free survival, whereas pathologically detectable nodes only did so if the tumor was at least 2 cm [24]; another using the same series showed that ultrasound was not sensitive for central compartment nodes but that these did not affect disease-free survival anyway [25]! Lateral nodes larger than 3 cm or numbering more than 5 appear to be particularly worrisome in one study of 621 patients [26]. The clinical significance of smaller nodes, however, remains unclear.
Ultrasound can also detect signs of local invasion, such as invasion of muscles [27], or other dangerous complications such as tracheal invasion [28] or invasion or encapsulation of local vessels such as the common carotid artery or internal jugular vein; invasion of vessels at the microscopic level (which is important histologically in discriminating follicular carcinoma from adenoma) is not detectable by ultrasound [27].
There is substantial evidence demonstrating that CT can detect nodes where US cannot. CT was shown to be more sensitive than US (77% vs. 62%) on a “per-level” analysis of 37 patients [29], was more sensitive in the central compartment in a study of 299 patients (though ultrasound was more accurate for extrathyroidal extension and intrathyroidal disease) [30], and added sensitivity to US for the central compartment in a study of 162 patients [31] and even to the lateral compartment (66% vs. 51%) in a study of 169 patients [32].
Postoperative Imaging
Ultrasound is often used after surgery as well. It is generally performed significantly after surgery (6–12 months), to allow postoperative changes time to resolve [3]. However, it has been used in the immediate postoperative setting or even during surgery, commonly in Europe, to check the lateral compartments if no preoperative ultrasound is available or if postablation scans or elevated thyroglobulin levels relative to postablation scans suggest spread of disease [20]. A study of 731 patients, all with lymph node involvement, who had ultrasound just after surgery and before radioiodine ablation showed a 95% NPV for persistent disease at 41 months, suggesting it may in fact be useful in high-risk cases [33]. Another study of 72 patients who had surgeon-performed ultrasound found a change in management in 57% of cases [10]. As such, it may show utility if there is a particular reason to suspect local disease has been missed on initial evaluation.
Recurrence
Ultrasound plays a key role in evaluation for recurrent disease; a negative ultrasound is required to define absence of persistent tumor and hence excellent response to therapy [2]. Visualization improves in the central compartment due to the removal of the thyroid [3]. It can be used to detect recurrent neck nodes and guide FNA for aspirations in cases of suspected recurrent disease.
Generally, it is recommended to wait at least 6 months after surgery to reexamine the neck with ultrasound [to allow postoperative changes to resolve] [34]. Current recommendations are to ultrasound the thyroid bed and central and lateral neck compartments at 6–12 months and then periodically depending on thyroglobulin levels and recurrence risk; low-risk patients with remnant ablation, a negative ultrasound, and negligible levels (<0.2 ng/mL or <1 ng/mL with TSH stimulation) do not need follow-up ultrasound [2].
Evidence for the use of ultrasound in the detection of recurrent disease is also quite strong. Ultrasound had a sensitivity of 94%, compared with 57% for thyroglobulin levels and 45% for WBS, in a study of 494 patients followed up over a mean of 54 months [35]. In 80 patients with papillary microcarcinoma (too small for RAI), ultrasound was able to identify three patients with nodal metastases, one of whom was thyroglobulin negative [36]. Notably, however, specificity may not be that high—many thyroid bed nodules never increase in size—a study of 191 patients found only 9% had an increase in size over 5 years [37], and another of 59 patients found recurrences were difficult to distinguish based on sonographic findings [38], and many can simply be followed.
As with the initial evaluation for metastatic disease, a cystic appearance or hyperechoic foci and peripheral vascularization are malignant, whereas a hyperechoic hilum and central vascularization suggest a benign node. A round shape or loss of the hilum and a hypoechoic appearance are not sufficient to indicate a biopsy is needed [39]. In addition to the sonographic appearance, clinical data should be considered as well. Risk of recurrence is higher with more metastatic nodes at initial presentation, as well as with extravascular extension [40] and with macroscopic nodal disease [8]; one study of 545 patients showed no significant increase in recurrence for microscopic nodal disease [41].
It is not clear if detection of metastases below 8–10 mm is of any clinical utility. If nodes are suspicious and at least 8–10 mm, fine-needle aspiration biopsy for cytology, with thyroglobulin measurement in the aspirate fluid, should be performed; thyroglobulin over 10 ng/mL in the aspirate is very suspicious (unless the patient has not been treated with RAI). Surgery is usually recommended with macroscopic disease only [3]. Smaller or less suspicious nodes can usually be monitored [2]. An example of a typical node is shown in Fig. 19.3.
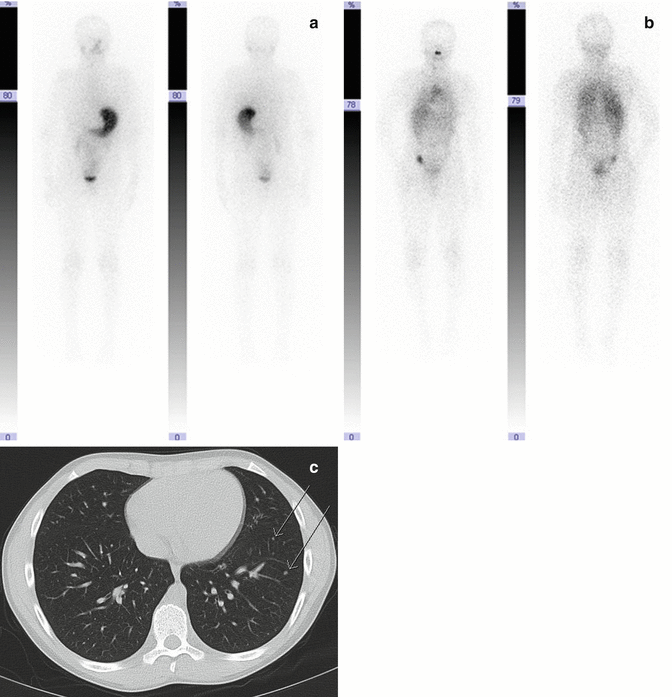

Fig. 19.3
A 10-year-old boy with preablation (a) and postablation (b) scans for thyroid carcinoma. Preablation scan shows no metastases, whereas postablation scan shows diffuse lung uptake. Chest CT (c) confirms miliary metastases (arrows)
If surgery is desired, present cutoffs for reoperation are 8 mm (short-axis diameter) in the central compartment and 10 mm in the lateral compartments [2]; even multiple revision surgeries have decreased thyroglobulin levels [41]. As with any surgery, other factors such as proximity to adjacent vital structures and (given the location in the neck) effects on vocal cord function, as well as patient comorbidities and primary tumor factors, should be considered as well [2]. In cases where reoperation is desired, surgery must be planned using medical imaging of some sort, in order to determine the location of the target. Together with CT, ultrasound can provide an effective presurgical map [31].
Nuclear Medicine
Primary Role: Detection of Residual and/or Metastatic Disease After Therapy and Detection of Disease in High-Risk Patients Afterward
There are two major nuclear medicine studies used in thyroid imaging, total body iodine (TBI) scintigraphy with radioactive iodine (123I or 131I) and positron emission tomography (PET) with fluorodeoxyglucose (FDG-PET). Whether to use TBI or FDG-PET depends largely on the anticipated level of differentiation of the cancer. More well-differentiated cancers tend to concentrate iodine but be less avid for FDG; poorly differentiated cancers tend to be less able to concentrate iodine (being less functional as thyroid tissue) but more avid for FDG (having increased anaerobic metabolic rate and upregulation of the GLUT1 glucose transporter) [45, 46]. As a result, cancers may be (and often are) visible on one scan and not on the other, and a previously iodine-avid, FDG-non-avid cancer may dedifferentiate in the process of metastasizing and become iodine-non-avid, FDG-avid cancer [47]. This is known as the “flip-flop” phenomenon, and there is a large amount of data demonstrating poorer prognoses in iodine-non-avid, FDG-avid cancers [48]. Generally, thus, TBI is used earlier in the course of disease when the tumor is assumed to still be iodine avid, and PET used later if it no longer appears to be taking up iodine.
FDG-PET/CT is best done with dedicated head and neck imaging, including images where additional time is spent scanning the head and neck and different reconstruction algorithms are used. This may add to the sensitivity and specificity for recurrent thyroid carcinoma in an otherwise negative workup [49].
Preoperative Staging
As previously mentioned, TBI is usually deferred until after thyroidectomy. While FDG-PET/CT is frequently used for initial staging of highly aggressive and rapidly spreading cancers such as lymphoma or melanoma, FDG-PET/CT is currently not recommended for initial staging of differentiated thyroid cancer [2], given the slow growth (and good differentiation) of most thyroid cancers, based on a study of 26 patients with initial staging of thyroid cancer where FDG-PET/CT showed no advantage over ultrasound and contrast-enhanced CT and showed a low sensitivity of 30% [50]. More recent work shows that a highly FDG-avid primary tumor is more likely to have metastases, but overall detectability remains low [51].
Postoperative Scanning: Preablation Scanning and Stunning
Following total thyroidectomy for well-differentiated thyroid cancer, the prognosis is related to the surgical pathology findings, as well as demographic factors such as age. The next step is often ablation of the residual functioning tissue (whether normal thyroid or tumor) with radioactive iodine (RAI). The dosage and other aspects of planning may depend on the amount (if any) of remnant tissue and whether any metastases are present. A common way of looking for remnant tissue and metastases is a preablation scan, where a low dose of RAI is given, enough to allow visualization of any iodine-avid metastases, but (hopefully) not enough to sensitivity of remaining thyroid tissue to RAI ablation.
According to ATA recommendations, postoperative (pretherapeutic) diagnostic RAI WBS can be useful if the extent of the thyroid remnant or residual disease cannot be ascertained from the surgical report or neck ultrasonography and when results may have clinical relevance (either altering decision to treat or dose of RAI). Recommended activity is 1.5–3 mCi of 123I or 1–3 mCi of 131I sodium iodide. In selected cases, SPECT/CT may be useful in further localizing areas of uptake [3].
There is substantial evidence that preablation TBI can alter clinical management, resulting in management changes in 25–53% of cases [52, 53], by detecting unsuspected regional metastases in 28–35% of cases and distant metastases in 4–8% of cases and by helping to differentiate between nodes and thyroid remnant in others [54].
However, there is concern that the prior diagnostic dose of radioactive iodine may decrease the effectiveness of the radioactive iodine ablation, an effect known as “stunning.” The evidence on this remains mixed and contradictory. There are studies showing that a prior 131I diagnostic scan decreases the effectiveness of subsequent 131I ablation [55], possibly with a dose-dependent effect with 3 mCi being worse than 1 mCi [56], although 1 mCi may still decrease the success of the ablation [57]. Other studies show no change at 1 mCi [58] or at 3–5 mCi [59] or even at 5 mCi. One potential solution is to use 123I for the preablation TBI, which theoretically should be at less risk for causing stunning as it is not a beta emitter and will therefore not deliver significant radiation dose to the thyroid. The literature is again split: one study suggests there is no difference in ablation failure rate between doing the diagnostic scan with 131I and 123I [60] (2 mCi of 131I), whereas yet another suggests a 3 mCi of 131I diagnostic scan does decrease ablation success rate vis-à-vis an 123I scan [61]. More recent work suggests apparent stunning on scans (a decrease in visible uptake) may not reflect outcome [62]. Guidelines suggest that therapeutic activity should be administered within 72 h of the diagnostic scan to avoid stunning [2], so a plan as to whether therapeutic activity is likely should be formulated ahead of time. More recent work suggests that, in addition to doing the scan within 3 days, waiting 1 week also decreases the effects of stunning [63].
SPECT/CT
SPECT/CT is an imaging technique used to better localize foci of iodine uptake. A rotating camera obtains 3-dimensional images of an area of the body in the fashion of a CT scanner, at the expense of half an hour of imaging time and a small amount of radiation from a low-dose CT used for image processing and anatomic localization. The current evidence suggests that performing SPECT/CT is in fact useful, at least as regards workup of a focus of activity detected on initial TBI, and it is currently recommended by guidelines for this purpose [3]. A study of 38 patients with a mixture of preablation and postablation scans showed SPECT/CT was able to resolve the cause of uncertain findings as nonmalignant in 80% of cases, avoiding unnecessary radioiodine therapy [64]. Another of 48 patients showed a preablation SPECT/CT scan changed postsurgical staging in 21% and proposed dose in 48% [65]. A study of 53 mostly preablation patients found incremental value in 48% of cases [66].
There is some argument for performing SPECT/CT as a routine part of a preablation scan, usually in higher-risk patients. A study of 79 patients who received SPECT/CT showed an increase in specificity from 68 to 100%, with sensitivity rising from 41 to 50% [67]. A prospective study of 320 patients who received SPECT/CT with their preablation scans showed a change in management in 29% of patients and a change in risk stratification in 15% [68]. A recent study of 83 patients showed preablation SPECT/CT had incremental value in 40% of cases [69]. However, even a low-dose CT carries at least some additional radiation, and this must be balanced against the likelihood of metastasis, particularly in low-risk patients who may not receive ablation. (The SPECT is done using the radiation from the original scan and does not carry any additional dose.)
Postablation TBI Scan
After the patient has received a therapeutic dose of radioactive iodine for ablation, ranging from 30–200 mCi depending on risk factors and disease burden, the activity from the ablation dose can be used for a post-therapy TBI scan. Because of the high administered dose, the postablation TBI may display metastases not visible on prior diagnostic doses. TBI may be useful after RAI remnant ablation or treatment, to inform disease staging and document the RAI avidity of any structural disease [2]. Images are usually taken 3–10 days after the administration of the radioactive iodine dose (the literature has ranged from 2 to 12).
There is evidence supporting routine utilization of TBI scans following radioablative therapy, primarily from single-center retrospective studies [70–75], mostly showing some variable increment of additional findings (anywhere from 6 to 50%) over and above a pretherapy scan. As many of these were performed on high-risk patients, the overall rate of residual or metastatic disease detected could be quite high, ranging from 20 to 84% [71, 73, 74], confirming the need for at least one scan in patients high risk enough to require ablation. An example of additional findings visible on the post-therapy scan is seen in Fig. 19.4.
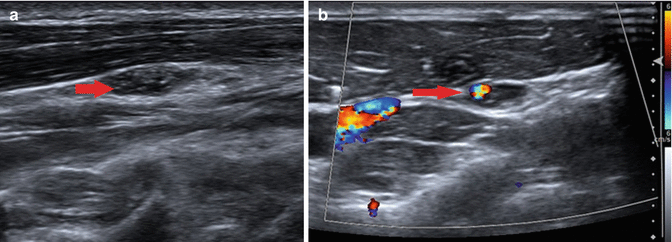

Fig. 19.4
1.2 × 1.1 cm level III node with microcalcifications (arrow, a) and peripheral vascularity (arrow, b), suspicious for malignancy. Patient was a 25-year-old male with prior history of thyroidectomy and radioiodine ablation. The decision was made to follow this node
One question under active investigation is whether SPECT/CT should be added to a post-therapy scan. The evidence here suggests that SPECT/CT is useful. Again, it is often performed on an as-needed basis for a detected focus of radioiodine uptake on a whole-body scan and usually shows improvement in diagnosis and/or management. SPECT/CT had a specificity of 95%, as opposed to 55% for planar TBI, in a study of 140 patients [76]. In one study of 41 patients with 53 scans with diagnostic uncertainty on planar TBI, it localized additional nodal disease in 29% and additional distant metastases in 21%, changing treatment decisions in 24% of patients [77]. Another study of 66 patients with locally advanced or metastatic disease, 23 of whom received SPECT/CT for inconclusive planar scans, found that SPECT/CT clarified the location or nature of metastases in 85 and 83% of patients, changing management in 47% of patients who received it [78]. Another study focusing specifically on inconclusive cases found changes in therapy in 25% of 25 cases [79]. Another study of 71 patients, mostly postablation, showed improvement in diagnosis in 57% of patients [80]. There is some evidence that imaging at 7 days may display metastases not visible at 3 [81].
However, for routine use, the case is somewhat less clear-cut, although it overall appears to favor the use of SPECT/CT. An early prospective study of 55 patients showed it clarified the 16% of indeterminate cases and correlated better with follow-up than planar SPECT [82]. A positive postablation SPECT/CT was a strong predictor of future recurrence (HR 65.2) in a single-center trial of 170 patients, with an estimated 78% sensitivity and 100% specificity [74]; another study of 81 patients showed a patient with a positive SPECT/CT was about ten times more likely to have abnormal TBI 5 months later; however, only 15% of patients with positive scans actually had uptake at 5 months [83]. A study of 109 patients, both postsurgical and with concern for recurrence, with intermediate- or high-risk disease who received SPECT/CT found that the need for additional cross-sectional imaging was reduced in 20% of patients and changed the ATA risk classification in 6% of cases. Non-iodine-avid lesions were detected on CT in 22% of cases, though some of these, such as subcentimeter lung nodules, were of unclear long-term significance [84]. Another study of 147 patients found that SPECT/CT changed staging in 6% of patients and management in 2% of patients [85]. Conversely, another study of 187 patients showed that SPECT/CT found additional nodal metastases in 9% of patients initially assumed to have only remnant uptake, whereas it proved there were no nodes in 40% of patients assumed to have nodes on planar scan [86]. Another study of 94 patients found that SPECT/CT improved localization in 21% and changed management in 23% of patients [87]. Another retrospective study similarly showed a change in management in 20% of patients [88]. Another of 93 patients showed a change in management in 20%, with frequent downstaging [89].
PET/CT in Initial Staging of Less Well-Differentiated Cancers
FDG-PET/CT is presently not recommended in well-differentiated thyroid cancer for initial staging. However, it may be useful for staging of more poorly differentiated cancers, both papillary and follicular cell with adverse histology and less differentiated types such as invasive Hürthle cell carcinomas [2]. A study of 286 patients with intermediate- to high-risk carcinoma showed a change in management in 14% of patients [92]. A study of 90 patients with either metastasized or extensive high-risk differentiated thyroid carcinoma found a total change in management in 21% of patients [93], and a 3-year follow-up found an 85–91% NPV for recurrence [94]. Another study of 197 patients with differentiated thyroid cancer showed a 67% sensitivity on a per-lesion basis, with a 70% sensitivity and 91% specificity for lateral neck nodes [95]. Another of 38 patients with aggressive histology, mostly tall cell or poorly differentiated, showed additional lesions on FDG-PET/CT over TBI scan in 41% of cases [96].
FDG-PET/CT has also shown to be effective for initial staging of Hürthle cell tumors; a study of 44 patients found 95% sensitivity and 95% specificity [97], and a smaller study of 17 patients for both initial and recurrent cancer showed 90% sensitivity [98]. For poorly differentiated thyroid cancer, which is intermediate between differentiated thyroid cancer and anaplastic thyroid cancer, there is suggestion that FDG-PET/CT is more avid, with uptake in 86% of a group of tumors [47] and 69% sensitivity in the previously mentioned group of 38 patients [96].
Given its avidity for more aggressive tumors, a positive FDG-PET/CT is a poor prognostic factor. A study of 202 thyroid cancer patients 6 months after initial treatment showed a positive PET/CT was a strong prognostic factor for decreased overall survival (HR 6.1) [99].
There is one study showing it may even be of use in determining whether radioiodine therapy will be effective: a study of 141 patients who received at least two ablations found FDG-avid metastases were less likely to demonstrate a good biochemical response and had poorer long-term survival [100].
Surveillance and Recurrence: Whole-Body Scintigraphy and SPECT/CT in Follow-Up
After surgery and RAI followed by TBI, the patient must be followed up to exclude recurrence. Whether this requires further TBI depends on the patient’s risk profile. After the first posttreatment TBI performed following RAI remnant ablation or adjuvant therapy, low-risk and intermediate-risk patients with an undetectable thyroglobulin on thyroid hormone with negative antithyroglobulin antibodies and a negative ultrasound do not require further evaluation with TBI [2, 101]. A meta-analysis of ten studies comprising 1959 patients suggested that a TSH-stimulated thyroglobulin test was sufficient to exclude recurrent disease (in the absence of antithyroid antibodies) and hence TBI was not needed [102]. A study of 315 patients had similar findings [103]. Indeed, the study is of low sensitivity if there is no uptake outside the thyroid bed on the initial scan [104].
However, for high-risk and higher intermediate-risk patients, diagnostic TBI (after either hormone withdrawal or rhTSH) 6–12 months after adjuvant radioiodine ablation therapy can be useful and can be done with 123I or low-activity 131I. Some recommended indications include uptake outside the thyroid bed on post-therapy TBI, large thyroid remnants which can obscure small nodes, and antithyroglobulin antibodies (making following the patient with thyroglobulin alone difficult) [2].
Total body iodine scans are also used for localization when a specific reason for suspecting recurrence exists, specifically if thyroglobulin becomes elevated (suggesting an iodine-concentrating, differentiated tumor). If thyroglobulin is elevated but TBI is negative, PET/CT is recommended to detect a dedifferentiated tumor. An example of iodine-negative, FDG-positive metastases is shown in Fig. 19.5.
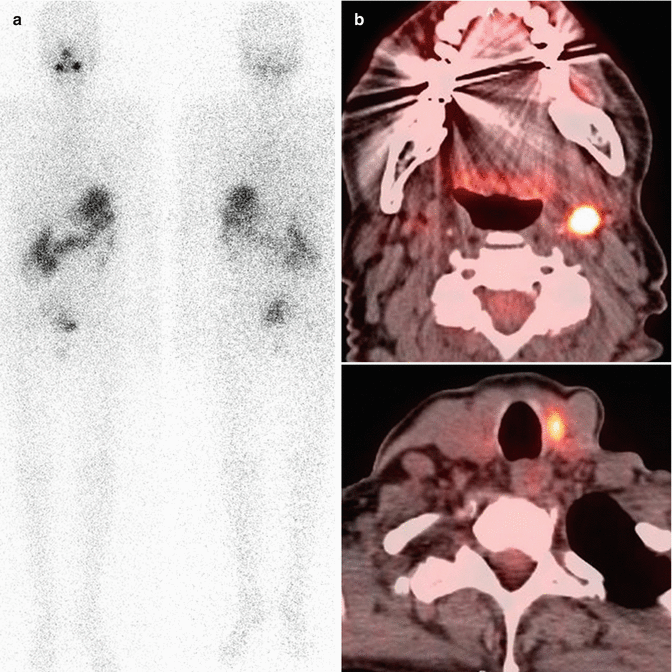

Fig. 19.5
This was a 1-year follow-up scan of a 59-year-old woman with thyroid carcinoma with nodal metastases who had been treated with surgery and radioiodine ablation. Iodine scan (a) is negative, but stimulated Tg was 24 ng/mL, and FDG-PET (b) shows local metastases (later confirmed by biopsy)
Most of the foregoing evidence on SPECT/CT of postablation scans also applies to recurrent disease. A few studies of the utility of SPECT specifically in recurrent disease have been done. At least one study of 87 patients found that SPECT/CT found evidence of disease in 9% of the total who would have otherwise gone untreated [105]. Another of 117 consecutive patients, most with recurrent disease, showed it avoided unnecessary treatment in 20% of cases, modified treatment in 36% of positive cases, and had at least some incremental value in 67% of cases [106].
PET/CT: TENIS and Beyond
If TBI is negative and serum Tg is over 10 ng/mL, FDG-PET/CT scanning is recommended [2]. (This is sometimes referred to as TENS or TENIS—thyroglobulin elevation with negative scintigraphy.) A 2007 meta-analysis of 20 studies of FDG-PET for recurrent thyroid cancer comprising 789 patients showed an overall 83% sensitivity (ranging from 50 to 100%) and 84% specificity (ranging from 42 to 100%) [107]; this was confirmed by a 2016 meta-analysis of 18 studies showing a 90% sensitivity and 80% specificity [108]. More recent studies confirm these values (84% sensitivity, 79% specificity) [109]. There is some evidence ultrasound may be more specific than TBI in the investigation of lateral neck nodes in the TENIS situation [110].
FDG-PET/CT is generally not recommended in patients with TSH-stimulated Tg < 10 ng/mL due to low sensitivity [10–30%], unless there are other factors that may cause a low thyroglobulin—for example, aggressive, poorly differentiated tumors that fail to secrete thyroglobulin or antithyroglobulin antibodies that prevent accurate measurement of thyroglobulin levels [2]. However, the latest meta-analysis of 34 studies of FDG-PET for recurrent thyroid cancer gave a pooled sensitivity and specificity of 79%, which included a variety of indications, so this may change with time [111]. It changes management in anywhere from 14 to 78% of cases of suspected recurrent thyroid cancer [112]. Another type of high-risk cancer for which FDG-PET/CT is also effective in the recurrent setting is Hürthle cell cancer; one of the few studies done specifically on recurrent Hürthle cell patients, on 17 patients, showed a 92% sensitivity and 80% specificity [113].
FDG-PET/CT scanning may also be considered as a prognostic tool in patients with metastatic disease and as an evaluation of posttreatment response following therapy of metastatic or locally invasive disease [2].
As with many other tumors, a high FDG uptake has prognostic value—largely negative, as it reflects a poorly differentiated carcinoma. Four hundred patients with DTC showed that FDG avidity was a strong negative predictor for survival [114]; another of 43 patients showed number of lesions and total lesion glycolysis were poor prognostic factors as well [115]. Another study of 80 patients with both FDG-PET/CT and radioiodine scans showed that FDG uptake was predictive for poor survival, whereas radioiodine uptake was prognostic for stable disease [116]. A negative FDG scan in the setting of TENIS syndrome appears to be a favorable prognostic indicator, although it complicates diagnosis [117]. One study of 54 patients with well-differentiated thyroid carcinomas in incidentally detected thyroid nodules, however, suggests it does not have prognostic value over and above standard factors such as age and stage [118], so this advantage may be restricted to the metastatic setting. Other metrics besides uptake such as heterogeneity have occasionally been used [119].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree