Imaging plays a central role in the detection, diagnosis, staging, and follow-up of renal cell carcinoma (RCC). Most renal masses are incidentally detected with modern, high-resolution imaging techniques and a variety of management options exist for the renal masses encountered today. This article discusses the role of multiple imaging modalities in the diagnosis of RCC and the imaging features of specific pathologic subtypes and staging techniques. Future directions in RCC imaging are presented, including dynamic contrast-enhanced and unenhanced techniques, as well as the development of novel tracers for positron emission tomography.
Renal cell carcinoma (RCC) accounts for about 3% of all adult cancers and 85% to 90% of all renal malignancies. Approximately 46,000 new cases of RCC were diagnosed in 2008. An estimated 50% to 60% of RCCs are found incidentally when diagnostic imaging is performed for an unrelated indication. As a result, renal tumor size and stage at presentation has steadily decreased in the United States in recent years, and the number of cases presenting with the classic triad of hematuria, flank pain, and an abdominal mass is declining.
The differential diagnosis of a renal mass includes both benign and malignant entities. Although RCC is the most common renal neoplasm and several pathologic subtypes of RCC show typical imaging features, tissue sampling or surgical resection is usually required to make a definitive diagnosis. However, imaging may permit the diagnosis of some benign lesions (eg, angiomyolipoma) and can help in the management of masses suspicious for RCC.
Modality-specific characterization of renal masses
Detection of vascularity in a renal mass is the most reliable finding to characterize a renal lesion as a neoplasm. Based on this premise, cross-sectional imaging studies are tailored primarily to detection of blood flow (Doppler ultrasound [US]) or enhancement after contrast administration (contrast-enhanced computed tomography [CT] and magnetic resonance imaging [MRI]) in a renal mass as an indicator of tumor vascularity. The characterization of cystic renal masses is also based on detection of complex solid components within the cyst. The presence of enhancing thick septa and/or nodules indicates a neoplasm, whereas cysts that contain more than a few thin septa may require imaging follow-up. A classification for characterization of cystic masses based on radiologic features (ie, the Bosniak classification), was originally developed to guide management of such lesions according to CT features, but may be applicable to lesions characterized by US and MRI as well.
US
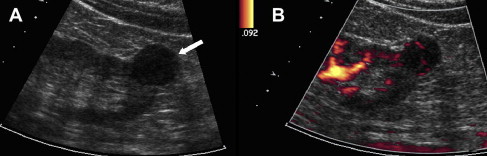
US is frequently used as the initial imaging modality to assess renal disorders because of the lack of radiation, low cost, and availability, and is particularly helpful for characterizing cystic renal masses. Benign simple cysts may be diagnosed confidently by US when lesions are anechoic and thin walled with posterior acoustic enhancement. Cystic lesions with more complex features may require imaging follow-up or surgical intervention. Doppler US evaluation yields additional important information regarding the vascularity of a lesion. A lesion of variable echogenicity and demonstrable blood flow is consistent with a solid neoplasm ( Fig. 1 ). Except for a highly echogenic lesion in which a diagnosis of angiomyolipoma is suspected (usually confirmed with CT or MRI), these findings usually indicate the need for surgical resection. Similarly, a vascularized mural nodule in an otherwise cystic lesion is highly suspicious for malignancy. Doppler US is also helpful for interrogation of patency of the renal vein and characterization of tumor thrombus, when present, by detecting blood flow within the thrombus.
CT
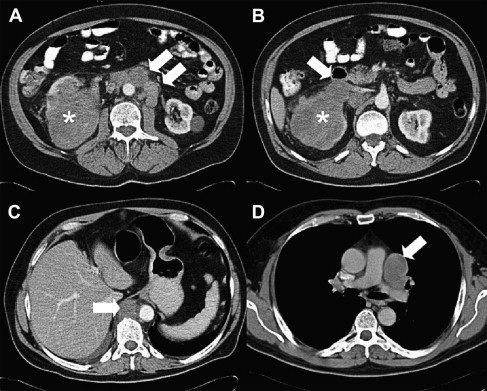
Contrast-enhanced, multiphasic, multidetector CT (MDCT) is the most commonly used imaging modality in the detection, characterization, and staging of RCC ( Fig. 2 ). MDCT assessment of renal mass improves with dynamic imaging during the corticomedullary, nephrographic, and excretory phases. The corticomedullary phase helps to differentiate the RCC subtypes based on tumor vascularity and provides valuable information about the vascular renal anatomy for planning a partial/radical nephrectomy. The nephrographic phase is optimal for detecting enhancement in a renal mass and improves the visualization of small renal masses. The excretory phase allows for delineation of the collecting system and its relationship with the renal mass. New MDCT protocols using dual-phase administration strategies of contrast may provide equivalent information with fewer acquisitions and, hence, decreased radiation to the patient. MDCT is also useful in the demonstration of macroscopic fat within a mass, which allows for the diagnosis of angiomyolipoma, a benign lesion included in the differential diagnosis of a renal mass. MDCT is also widely used for surveillance following nephrectomy and percutaneous thermal ablation, to evaluate metastatic disease, and to monitor treatment response.
MRI
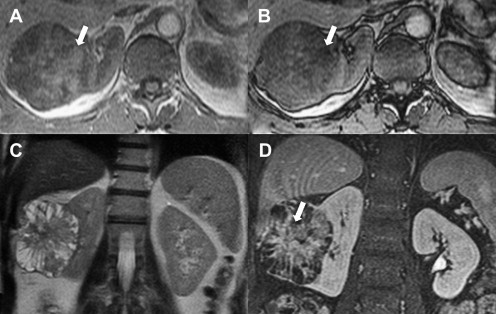
MRI is a modality suited to renal mass characterization because of its superior soft tissue contrast. Noncontrast T1-weighted and T2-weighted MRI, including chemical shift imaging, permit detection of both bulk fat (as in benign angiomyolipomas) and intravoxel fat (as in clear cell RCC [ Fig. 3 ]) or angiomyolipomas with minimal fat. Furthermore, the exquisite sensitivity of MRI for small amounts of gadolinium-based contrast agents is particularly suited for the detection of enhancement within a renal lesion (see Fig. 3 ), which can be facilitated with the generation of subtracted images. When paired with high-resolution three-dimensional MRI, the sensitivity to detect solid, nodular enhancing components within a renal cyst is unparalleled; when present, these have excellent positive predictive value for malignancy, correlating with RCC in 95% of such lesions. MRI is also useful in the locoregional staging through the assessment of regional lymph nodes and is superior to CT for the detection of tumor thrombus in the renal vein and inferior vena cava.
Positron Emission Tomography
Positron emission tomography (PET) with 18 F-labeled fluoro-2-deoxy-2-glucose (FDG) provides an alternative to contrast-enhanced approaches by showing metabolically active disease, and is helpful for the diagnosis and follow-up of patients with many types of cancer. Although RCC can have intense FDG uptake, uptake is often mild and similar in intensity to adjacent renal parenchyma. Furthermore, excreted radiotracer in the renal collecting system may obscure renal masses. Therefore, a negative study does not exclude malignancy. Published studies report a broad range of accuracy rates for primary tumors and staging, likely because of variability in the degree of tumor uptake, likely secondary to differences in primary tumor differentiation, patient populations, and low accuracy in comparison to CT. To date, the role of FDG-PET in the initial detection and diagnosis of RCC is therefore limited. However, FDG-PET seems to show some promise for the detection of distant metastases ( Fig. 4 ) and local recurrence, and may be occasionally complementary to other cross-sectional imaging techniques. A study by Majhail and colleagues suggested that, despite high specificity, FDG-PET is not a sensitive imaging modality for the evaluation of metastatic RCC and may not adequately characterize small metastatic lesions.
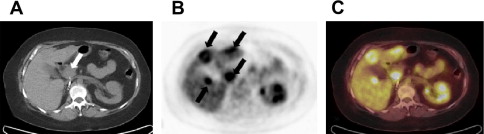
FDG-PET may be useful as a biomarker of metabolic tumor response to therapy, such as with sunitinib therapy in both soft tissue and skeletal lesions. Metabolic response by FDG-PET after 1 cycle of sunitinib therapy was predictive of later response as evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) on CT. Other investigators found that PET response at 16 weeks predicts outcome, which is not the case at 4 weeks. Recent reports have indicated that intense FDG uptake may indicate a worse outcome compared with less intensely FDG-avid RCC lesions, and that FDG-PET may be useful in the postoperative surveillance of advanced RCC.
Imaging findings in distinct histopathologic subtypes of RCC
The World Health Organization classification of RCC includes clear cell, papillary, chromophobe, collecting duct, medullary, and unclassified categories. Imaging and pathologic features vary among the RCC subtypes, and pathologic diagnosis is important because of differences in biologic behavior, response to current therapies, and prognosis.
Clear cell is the most common RCC subtype, accounting for about 70% to 75% of cases, characterized by glycogen-rich and lipid-rich clear cells histologically. Most sporadic clear cell RCCs show inactivating somatic mutations of the von Hippel-Lindau gene. On CT imaging, clear cell RCC is typically hypervascular and heterogeneous because of central necrosis ( Fig. 5 ), hemorrhage, and/or foci of calcification. On MRI, typical features of clear cell RCC may include the loss of signal on opposed-phase images caused by intracellular lipid, a rim of tumor surrounding central necrosis and/or hemorrhage, and avid enhancement, particularly in the corticomedullary phase, in contrast with other RCC subtypes. A pseudocapsule of low signal intensity on T1-weighted images and T2-weighted images may be present, and disruption of this pseudocapsule is more common in high-grade clear cell neoplasms. Predominantly cystic masses with fluid contents similar in appearance to that of simple cysts on T2-weighted imaging, but with variable amounts of enhancing thick septae and nodularity, correlate with low-grade clear cell histology. Vascular involvement, with extension into the renal vein and inferior vena cava (IVC), may occur and, together with the presence of retroperitoneal collaterals and tumor necrosis, are predictive of high-grade histology in clear cell RCC. Hematogenous metastases primarily involve the lungs, liver, and bones. Lymph node metastases are reported in about one-sixth of cases. In general, clear cell RCC has a poorer prognosis than other histologic subtypes.
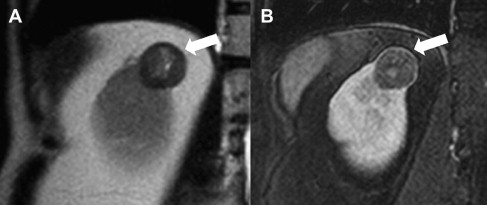
Papillary RCC is the second most common histologic subtype, accounting for 10% to 15% of cases, with 2 subtypes described based on histologic appearance and behavior. However, distinction of the 2 papillary subtypes at imaging is usually not possible. On CT imaging, these tumors are often homogeneous and hypoenhancing compared with adjacent renal parenchyma. On MRI, papillary RCC often manifests as a small peripheral mass that is hypointense on T2-weighted images because of intratumoral hemosiderin. Papillary RCC frequently undergoes internal hemorrhage leading to another characteristic appearance on MRI: cystic mass with hyperintense fluid content on T1-weighted images and enhancing papillary projections arising from the wall of the cyst. Dynamic contrast-enhanced MRI shows low-level, progressive enhancement ( Fig. 6 ), which allows for accurate differentiation from the characteristically hypervascular clear cell carcinomas. Metastasis from papillary RCC shows similar features and tends to be hypovascular. Compared with clear cell RCC, tumor extension into the renal vein and IVC is less common; visceral metastasis is less frequent, with lung most common; and nodal involvement is more prevalent.
Chromophobe RCC accounts for 5% of RCC. Birt-Hogg-Dube syndrome is associated with chromophobe RCC and oncocytomas. On CT imaging, these tumors tend to be large and homogeneous. Chromophobe RCCs typically lack hemorrhage and necrosis pathologically and this reflects in their imaging appearance; these tumors, unlike clear cell RCC, can grow to a large size without undergoing central necrosis. Perinephric extension and renal vein involvement occurs infrequently. On MRI, chromophobe RCCs may be heterogeneous in appearance, similar to clear cell tumors, but enhancement tends to be more moderate, between that of clear cell and papillary subtypes. Recently, a characteristic segmental enhancement inversion in contrast-enhanced MDCT and MRI has been described both for oncocytomas and chromophobe RCC, but is not present in other RCC subtypes or angiomyolipoma. This enhancement pattern is characterized by increased enhancement in a segment within the mass during the corticomedullary phase that becomes less enhanced during the later phases, whereas a less-enhanced segment during the corticomedullary phase becomes hyperenhanced during the excretory phase. Patients with chromophobe RCC have a greater propensity for liver metastases compared with other subtypes.
Other uncommon types of RCC include collecting duct (Bellini) tumors, medullary RCC, multilocular cystic RCC, and unclassified types. Collecting duct tumors are central and infiltrative, and are associated with a poor prognosis. Medullary RCC are indistinguishable from collecting duct tumors but occur characteristically in young patients with sickle cell trait. Multilocular cystic RCC are predominately cystic masses with multiple septae and carry excellent prognosis. By definition, these neoplasms do not contain solid elements within the cyst, although mildly thickened, irregular septations are commonly seen. It is challenging to differentiate these from benign complex cysts and/or multilocular cystic nephroma by imaging. Enhancing mural or septal nodules are a feature of the more common cystic subtype of clear cell RCC. Typical imaging features of unclassified types have not yet been described.
Overall, there are limited data about the ability to predict the histologic diagnosis based on imaging findings. In a retrospective study, Sheir and colleagues found different levels of enhancement on contrast-enhanced CT examinations, with the highest accuracy for the diagnosis of clear cell subtype when using a cutoff value in attenuation of 83.5 Hounsfield units (HU) on images acquired during the corticomedullary phase and 64.5 HU for images acquired during the excretory phase, respectively. In another retrospective analysis of 198 renal masses evaluated by CT, including some benign lesions, Zhang and colleagues found that clear cell RCC was characterized by a mixed enhancement pattern with areas of hyperenhancement and low attenuation, whereas other types of RCC were characterized by homogeneous or peripheral enhancement. A classification based on the appearance of malignant tumors on MRI, or MRI phenotype, was proposed by Pedrosa and colleagues. The reported sensitivity and specificity for the diagnosis of clear cell subtype using this classification were of 93% and 75%, respectively. Similarly, the sensitivity and specificity for the diagnosis of papillary subtype were 80% and 94%, respectively. Sun and colleagues found excellent diagnostic accuracy to differentiate clear cell from papillary histology on dynamic contrast-enhanced MRI, with a reported sensitivity and specificity of 93% and 96% respectively, when using a cutoff of 84% change in signal intensity during the corticomedullary phase compared with precontrast images.
Hereditary RCC and Other Associations
Hereditary RCC syndromes refer to multisystem syndromes with an increased predisposition to multiple tumors, including RCCs. Ten hereditary RCC syndromes have been described, all with autosomal dominant inheritance of increased cancer susceptibility. The most commonly known syndromes include von Hippel-Lindau disease, hereditary papillary RCC, and tuberous sclerosis. A syndrome may be suspected when there is a family or individual history of renal tumors, bilateral or multiple tumors, or early onset. MRI surveillance and follow-up may be favored in these patients, to avoid the cumulative radiation associated with multiple CT examinations in a young patient, although specific follow-up schedules have not been established. Detailed delineation of the anatomy and tumor extent is crucial in these patients to facilitate nephron-sparing surgical resections because they frequently require repeated surgical procedures during their life span.
Imaging findings in distinct histopathologic subtypes of RCC
The World Health Organization classification of RCC includes clear cell, papillary, chromophobe, collecting duct, medullary, and unclassified categories. Imaging and pathologic features vary among the RCC subtypes, and pathologic diagnosis is important because of differences in biologic behavior, response to current therapies, and prognosis.
Clear cell is the most common RCC subtype, accounting for about 70% to 75% of cases, characterized by glycogen-rich and lipid-rich clear cells histologically. Most sporadic clear cell RCCs show inactivating somatic mutations of the von Hippel-Lindau gene. On CT imaging, clear cell RCC is typically hypervascular and heterogeneous because of central necrosis ( Fig. 5 ), hemorrhage, and/or foci of calcification. On MRI, typical features of clear cell RCC may include the loss of signal on opposed-phase images caused by intracellular lipid, a rim of tumor surrounding central necrosis and/or hemorrhage, and avid enhancement, particularly in the corticomedullary phase, in contrast with other RCC subtypes. A pseudocapsule of low signal intensity on T1-weighted images and T2-weighted images may be present, and disruption of this pseudocapsule is more common in high-grade clear cell neoplasms. Predominantly cystic masses with fluid contents similar in appearance to that of simple cysts on T2-weighted imaging, but with variable amounts of enhancing thick septae and nodularity, correlate with low-grade clear cell histology. Vascular involvement, with extension into the renal vein and inferior vena cava (IVC), may occur and, together with the presence of retroperitoneal collaterals and tumor necrosis, are predictive of high-grade histology in clear cell RCC. Hematogenous metastases primarily involve the lungs, liver, and bones. Lymph node metastases are reported in about one-sixth of cases. In general, clear cell RCC has a poorer prognosis than other histologic subtypes.
Papillary RCC is the second most common histologic subtype, accounting for 10% to 15% of cases, with 2 subtypes described based on histologic appearance and behavior. However, distinction of the 2 papillary subtypes at imaging is usually not possible. On CT imaging, these tumors are often homogeneous and hypoenhancing compared with adjacent renal parenchyma. On MRI, papillary RCC often manifests as a small peripheral mass that is hypointense on T2-weighted images because of intratumoral hemosiderin. Papillary RCC frequently undergoes internal hemorrhage leading to another characteristic appearance on MRI: cystic mass with hyperintense fluid content on T1-weighted images and enhancing papillary projections arising from the wall of the cyst. Dynamic contrast-enhanced MRI shows low-level, progressive enhancement ( Fig. 6 ), which allows for accurate differentiation from the characteristically hypervascular clear cell carcinomas. Metastasis from papillary RCC shows similar features and tends to be hypovascular. Compared with clear cell RCC, tumor extension into the renal vein and IVC is less common; visceral metastasis is less frequent, with lung most common; and nodal involvement is more prevalent.
Chromophobe RCC accounts for 5% of RCC. Birt-Hogg-Dube syndrome is associated with chromophobe RCC and oncocytomas. On CT imaging, these tumors tend to be large and homogeneous. Chromophobe RCCs typically lack hemorrhage and necrosis pathologically and this reflects in their imaging appearance; these tumors, unlike clear cell RCC, can grow to a large size without undergoing central necrosis. Perinephric extension and renal vein involvement occurs infrequently. On MRI, chromophobe RCCs may be heterogeneous in appearance, similar to clear cell tumors, but enhancement tends to be more moderate, between that of clear cell and papillary subtypes. Recently, a characteristic segmental enhancement inversion in contrast-enhanced MDCT and MRI has been described both for oncocytomas and chromophobe RCC, but is not present in other RCC subtypes or angiomyolipoma. This enhancement pattern is characterized by increased enhancement in a segment within the mass during the corticomedullary phase that becomes less enhanced during the later phases, whereas a less-enhanced segment during the corticomedullary phase becomes hyperenhanced during the excretory phase. Patients with chromophobe RCC have a greater propensity for liver metastases compared with other subtypes.
Other uncommon types of RCC include collecting duct (Bellini) tumors, medullary RCC, multilocular cystic RCC, and unclassified types. Collecting duct tumors are central and infiltrative, and are associated with a poor prognosis. Medullary RCC are indistinguishable from collecting duct tumors but occur characteristically in young patients with sickle cell trait. Multilocular cystic RCC are predominately cystic masses with multiple septae and carry excellent prognosis. By definition, these neoplasms do not contain solid elements within the cyst, although mildly thickened, irregular septations are commonly seen. It is challenging to differentiate these from benign complex cysts and/or multilocular cystic nephroma by imaging. Enhancing mural or septal nodules are a feature of the more common cystic subtype of clear cell RCC. Typical imaging features of unclassified types have not yet been described.
Overall, there are limited data about the ability to predict the histologic diagnosis based on imaging findings. In a retrospective study, Sheir and colleagues found different levels of enhancement on contrast-enhanced CT examinations, with the highest accuracy for the diagnosis of clear cell subtype when using a cutoff value in attenuation of 83.5 Hounsfield units (HU) on images acquired during the corticomedullary phase and 64.5 HU for images acquired during the excretory phase, respectively. In another retrospective analysis of 198 renal masses evaluated by CT, including some benign lesions, Zhang and colleagues found that clear cell RCC was characterized by a mixed enhancement pattern with areas of hyperenhancement and low attenuation, whereas other types of RCC were characterized by homogeneous or peripheral enhancement. A classification based on the appearance of malignant tumors on MRI, or MRI phenotype, was proposed by Pedrosa and colleagues. The reported sensitivity and specificity for the diagnosis of clear cell subtype using this classification were of 93% and 75%, respectively. Similarly, the sensitivity and specificity for the diagnosis of papillary subtype were 80% and 94%, respectively. Sun and colleagues found excellent diagnostic accuracy to differentiate clear cell from papillary histology on dynamic contrast-enhanced MRI, with a reported sensitivity and specificity of 93% and 96% respectively, when using a cutoff of 84% change in signal intensity during the corticomedullary phase compared with precontrast images.
Hereditary RCC and Other Associations
Hereditary RCC syndromes refer to multisystem syndromes with an increased predisposition to multiple tumors, including RCCs. Ten hereditary RCC syndromes have been described, all with autosomal dominant inheritance of increased cancer susceptibility. The most commonly known syndromes include von Hippel-Lindau disease, hereditary papillary RCC, and tuberous sclerosis. A syndrome may be suspected when there is a family or individual history of renal tumors, bilateral or multiple tumors, or early onset. MRI surveillance and follow-up may be favored in these patients, to avoid the cumulative radiation associated with multiple CT examinations in a young patient, although specific follow-up schedules have not been established. Detailed delineation of the anatomy and tumor extent is crucial in these patients to facilitate nephron-sparing surgical resections because they frequently require repeated surgical procedures during their life span.
Staging, management, and surveillance
Because surgery plays a central role in the management of RCC and many cancers in general, it is logical that staging systems have incorporated detailed categorization of locoregional disease extent to facilitate resection. In the 1960s, the Robson classification was developed to stage renal cell cancers, a widely used system with a focus on the degree of primary tumor spread into adjacent tissues, in which stage I tumors were confined to the renal capsule; stage II tumors extended to the perirenal fat or ipsilateral adrenal gland; stage III tumors had vascular (A) or nodal (B) extension, or both (C); and stage IV indicated distant disease. The Robson classification has been replaced by tumor, node, metastasis (TNM) staging, commonly used in cancer staging today worldwide. The obvious advantage of the TNM system maintained by the International Union Against Cancer is the widespread, standardized application of this system allowing disease study and continual refinement and evolution of the staging system in response to new developments. From a practical point of view, the TNM classification provides important prognostic information about the likelihood of disease progression.
In the seventh edition (2010) of the TNM classification ( Table 1 ), the T staging indicating the size of the primary tumor was defined as follows: T1 to 2 disease is organ confined (T1a≤4 cm, T1b 4–7 cm, T2a 7–10 cm, T2b>10 cm). Stage T3a indicates extension to the perinephric tissue, renal sinus, or renal vein, whereas T3b tumors extend to the infradiaphragmatic IVC and T3c tumors extend to the supradiaphragmatic IVC or into the vessel wall at any level. T4 disease indicates extension beyond Gerota fascia or directly into the ipsilateral adrenal gland. N1 indicates disease in regional lymph node(s) (eg, adjacent to the renal vasculature, para-aortic for left-sided masses or paracaval for right-sided masses). M1 disease represents distant disease and includes nonregional lymph nodes. Recent updates in the seventh edition include the division of T2a and T2b disease, incorporating renal vein and perinephric involvement into T3a, and advancing adrenal involvement to T4 disease. A recent validation study of the seventh edition indicates opportunities for further improvements in TNM staging, with overlap in prognosis associated with some stages and heterogeneous outcomes in others.
| Definitions of TNM | |
|---|---|
| Primary tumor | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor ≤7 cm in greatest dimension; limited to the kidney |
| T1a | Tumor ≤4 cm in greatest dimension; limited to the kidney |
| T1b | Tumor >4 cm but ≤7 cm in greatest dimension; limited to the kidney |
| T2 | Tumor >7 cm in greatest dimension; limited to the kidney |
| T2a | Tumor >7 cm but ≤10 cm in greatest dimension; limited to the kidney |
| T2b | Tumor >10 cm; limited to the kidney |
| T3 | Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota fascia |
| T3a | Tumor grossly extends into the renal vein or its segmental (muscle-containing) branches, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota fascia |
| T3b | Tumor grossly extends into the vena cava below the diaphragm |
| T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava |
| T4 | Tumor invades beyond Gerota fascia (including contiguous extension into the ipsilateral adrenal gland) |
| Regional lymph nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in regional lymph node(s) |
| Distant metastasis | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
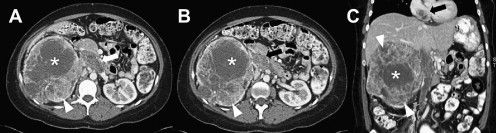
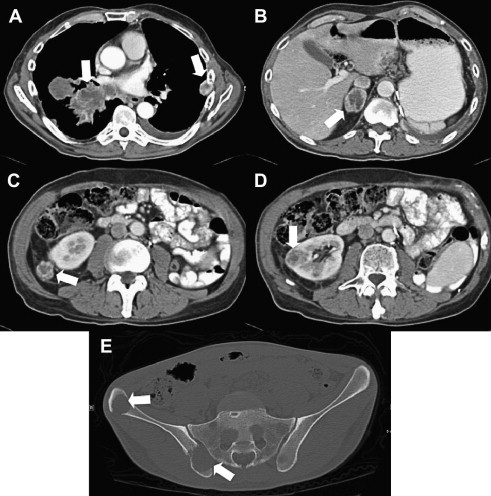
CT and MRI offer noninvasive preoperative staging and surgical planning in patients with renal masses suspicious for RCC. The role of preoperative imaging is to identify tumor features that render a patient amenable to a particular treatment approach and to assess for distant disease ( Fig. 7 ). Small renal masses may be managed with nephron-sparing partial nephrectomy, thermal ablation, or, in some cases, active surveillance. Therefore, important components of the radiologic report include the size and position of the renal mass within the kidney, specifically whether it is largely exophytic or if it extends into the renal sinus, for appropriate surgical planning. Evidence and extent of renal vein/IVC involvement must be described. The presence and location of suspicious adenopathy should also be detailed.
Some small renal tumors (particularly those <4 cm) are also amenable to alternative, minimally invasive, thermal ablative therapies, specifically cryoablation and radiofrequency ablation (RFA). Cryoablation involves tissue freezing to temperatures to −20 to −50°C with visible ice ball formation, whereas RFA involves tissue heating to temperatures greater than 50°C with coagulation necrosis. These ablation strategies can be applied via open, laparoscopic, or percutaneous approaches, although small exophytic masses that spare the renal hilum, located away from the main renal vasculature and ureter, are best suited to the percutaneous technique. Percutaneous ablations are performed using US, CT, or magnetic resonance (MR) guidance ( Fig. 8 ). Some advantages of percutaneous ablations include the least invasive approach, tamponade effect of Gerota fascia, and, for cryoablation specifically, direct visualization of the entire ice ball via CT or MRI, consistent pattern of tissue ablation, and associated decreased risk of injury to the urinary tract or other adjacent tissues. In a meta-analysis of percutaneous and surgical approaches to ablation, major complications were decreased in percutaneously treated patients, with equally effective treatment in the groups. However, more than 1 treatment is sometimes needed for effective treatment in percutaneously treated patients with residual viable tumor after an initial ablation. Imaging features of successful ablation and long-term efficacy remain undefined, although tumor shrinkage with time and absence of internal enhancement have been associated with effective ablation. However, long-term imaging follow-up is necessary to monitor potential recurrent disease.