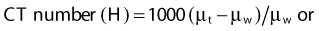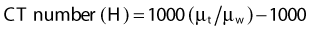8 Imaging in Radiation Oncology*
Accurate, patient-specific anatomic information is a prerequisite for planning and implementing the delivery of radiation to the entire extent of the malignancy while minimizing exposure to critical structures. For this reason, anatomic images are of utmost importance in radiotherapy. In fact, most of the significant recent advances in radiation oncology have resulted from developments in imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy imaging (MRSI), positron emission tomography (PET), and digital planar image receptors. With information from the new imaging modalities, it is now possible to define treatment volumes and critical structures with great precision, thus reducing marginal misses and irradiation of normal tissues. Such capabilities may permit higher tumor doses, potentially leading to improved local control, while maintaining the same level of normal tissue morbidity or even reducing it.1–4
Generation of Images
Computed Tomography
Principles of Operation
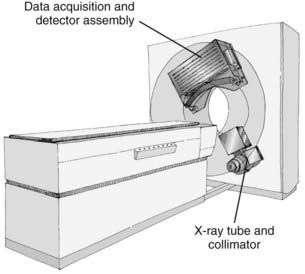
CT is an x-ray imaging technique used to visualize thin slices of the body. The first commercial CT scanner was developed by Sir Godfrey Hounsfield of the EMI Corporation in 1972. Alan Cormack developed an accurate mathematical technique to reconstruct images from x-ray projections. Hounsfield and Cormack received the 1979 Nobel Prize in medicine for their efforts. Modern scanners employ a pure rotation motion and require only 0.4 to 1.0 second per rotation to acquire the data (Fig. 8-1). Multislice detector arrays now allow as many as 320 slices to be acquired in one 0.35 second rotation.
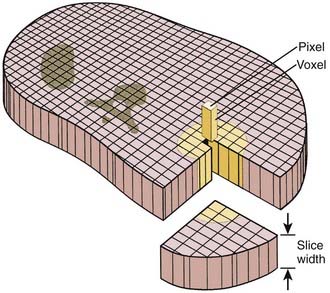
A thin beam of x-rays of about 120 kilovolts (kV), in the fan-beam geometry, is incident on the patient transversely. The transmitted x-rays are detected by an array of many solid-state detectors. Concomitantly, the x-ray source and the detector array are rotated through an angular range from 180 to 360 degrees. The detected x-ray transmission factors are input data to a computer program, the so-called reconstruction algorithm, which produces an output matrix (usually 512 × 512) of digital values. Current scanners using workstations and the filtered-back projection algorithm can reconstruct an image in a fraction of a second. Each element of the matrix represents a small area, or pixel; the product of a pixel area and the slice thickness constitutes a volume element, or voxel (Fig. 8-2). The digital value reconstructed for each pixel (or voxel) represents the linear attenuation coefficient (approximately equivalent to the electronic density) of that volume. The digital values are converted to gray-scale levels for display on a video monitor screen or are used as input to a PACS workstation or to a laser imager for hard-copy output on film. The contrast of the image may be adjusted by window and level settings that determine the range of attenuation values displayed within the gray-scale range of the output device.
where µt is the linear attenuation coefficient of the tissue element in that pixel, and µw is the linear attenuation coefficient of water at the effective energy of the x-ray beam. With this definition, water has a CT number of 0 H, air of −1000 H, and dense bone of more than 1000 H. In general, CT values for soft tissues range from −100 to +100 H.
Uses in Radiation Oncology and Limitations
Computed tomographic images are usually acquired with a 120 to 140 kV x-ray beam (an effective energy of 70 to 80 keV), which undergoes a significant number of photoelectric interactions, particularly in bone. Therefore, a nonlinear conversion table must be used to obtain electron densities for dose calculations relevant to the much higher energy treatment photons that undergo mainly Compton interactions. Several authors have developed an empiric relationship between CT numbers in H units and electron densities normalized to 1.0 for water.5 This relationship is expressed as two or three linear regions and stored in a lookup table (LUT). Phantoms that contain multiple objects of a known electron density for converting CT numbers are commercially available.
Recent Developments
Faster and more accurate CT examinations have become possible with the introduction first of the single-slice and then of the multi-slice spiral, or (more properly) helical, scanners. Volumetric scan data is acquired by having the table move continuously relative to the gantry, concomitant with the continuous rotation of the x-ray tube and detector array around the patient. Multislice units may acquire the data for as few as 4 or as many as 320 slices during a single 0.35 second rotation.6 State-of-the-art multislice CT scanners can obtain isotropic data sets with voxels of dimension as small as 0.5 mm. These scanners can acquire the data for a full set of images in times well below 1 minute, usually during one breath hold. For radiotherapy application, however, one must consider the possibility that patient anatomy during a breath hold may not be in its “average” location during radiation treatment.
To address the limitations of CT gantry aperture on the ability to set up patients with immobilization devices, commercial scanners with 80 to 85 cm apertures have become available. In connection with respiration-gated radiation therapy, in which dose delivery from the treatment accelerator occurs at a preselected interval in the respiratory cycle while the patient breathes normally, different means of synchronizing CT acquisition with respiration have been introduced. In one method, a respiration monitor system triggers the scanner to acquire a single axial image while the patient breathes normally, followed by indexing of the table to the next position; the process is repeated until the volumetric set of images is obtained.7 A more widely used approach is the acquisition of volumetric data concurrently throughout the respiration cycle, for the purposes of obtaining information on anatomical motion and more accurately accounting for it in the treatment plan.8,9 In this approach, axial images are acquired continuously while the respiration signal is simultaneously recorded; the images are then retrospectively sorted according to the respiration phase to form volumetric sets at different phases.
The use of CT for treatment verification is discussed in the section on In-Room CT-Based Systems.
Use and Limitations of Computed Tomographic Scout View
Digital projection images, the so-called scout view, can be obtained from CT scanners by translating the patient relative to the x-ray tube and detector array (Fig. 8-3). Although the x-ray tube may be oriented at any angle, the most common arrangement is in either the anterior-posterior (AP) or the lateral direction. The data obtained are a two-dimensional (2-D) matrix of relative transmission values in an arbitrary numeric range that may differ from one type of scanner to another. Adjustment of the window and level controls provides an image of appropriate brightness and contrast. The scout view is often useful in determining the extent and spacing of axial scans. In addition, in the hard-copy output, the positions of the axial images can be annotated on the scout views, relative to a reference position selected during the patient setup.
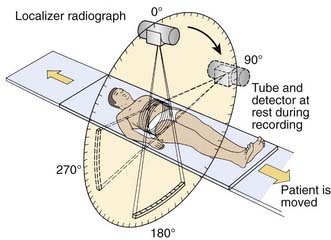
FIGURE 8-3 • Schematic drawing illustrating the acquisition of a localizer radiograph, or scout view.
(From Krestel E [ed]: Imaging systems for medical diagnostics: fundamentals and technical solutions, Berlin, 1990, Siemens, p. 434.)
Magnetic Resonance Imaging
Principles of Operation
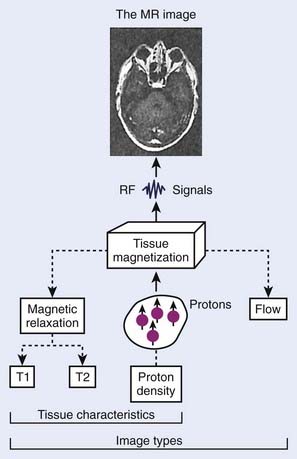
The phenomenon of nuclear magnetic resonance (NMR) was first described by Purcell and Bloch, the 1952 winners of the Nobel Prize in physics. Early work on the use of NMR for imaging was done by Lauterbur, Damadian, and others. Nuclei with a magnetic moment, usually those with odd numbers of protons or neutrons, when placed in a strong magnetic field, can be excited from their ground or lowest energy state to a higher state by a pulse from a second and weaker magnetic field that is varying in radiofrequency. When these nuclei return to the ground energy state, they emit radiofrequency energy that can be detected by very sensitive wire coils and appropriate electronics. Many commercial MRI units use a 0.5 to 1.5 tesla (T) magnetic field produced by a superconducting magnet and radiofrequency signals of 20 to 65 MHz for studies of the distribution of hydrogen nuclei (protons) in the body. Additional electromagnets are used to localize the region where resonance takes place (x-, y-, and z-gradient coils) and remove inhomogeneities in the main magnetic field (shim coils) (Fig. 8-4).
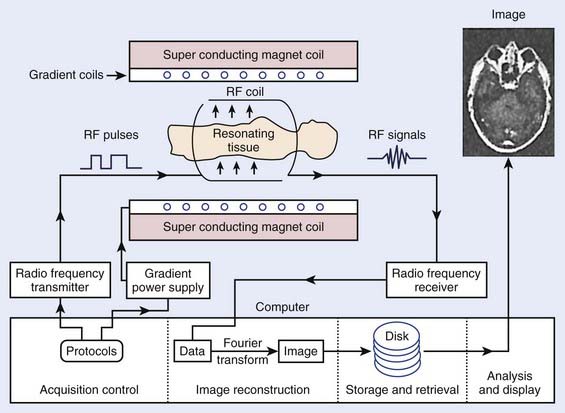
FIGURE 8-4 • Typical magnetic resonance imaging system. RF, Radiofrequency.
(From Sprawls P: Physical principles of medical imaging, ed 2, Madison, WI, 1993, Medical Physics Publishing.)
Typical magnetic resonance (MR) images contain a matrix of 256 × 256 values, a factor of 2 less than CT images in each dimension. Each pixel contains a complex combination of information about hydrogen nuclei density (ρ), spin-lattice relaxation time (T1), and spin-spin relaxation time (T2). The relaxation times are a measure of the time required for the disappearance of the signal caused by nuclei returning from an excited to an unperturbed energy state, or for dephasing of the initially aligned and precessing nuclear spins. Radiofrequency pulse sequences can be designed to enhance the dependence of the image on the values of any of the three parameters and improve the contrast for various tumors or other abnormal tissue variations. The appearance of MR images may be further affected by blood flow or by injection of contrast agents such as gadolinium compounds. MR image data may be obtained for a three-dimensional (3-D) volume or for any arbitrary plane within the volume. MRI offers the possibility of excellent discrimination of certain tumors with high contrast, the ability to select arbitrary planes for imaging, and good resolution (Fig. 8-5).
Uses and Limitations
MR images often provide better soft-tissue contrast than CT, thus improving discrimination between tumor and normal tissue in some disease sites. Small differences in T1 and T2 can be exploited by manipulating imaging parameters to enhance the contrast among different tissues. In addition, by using the three orthogonal field gradients independently or in combination, the image plane can be oriented in a direction that best displays the extent of a particular tissue. Functional MRI of the brain can identify speech, visual, and sensory areas to avoid when treating intracranial lesions.10
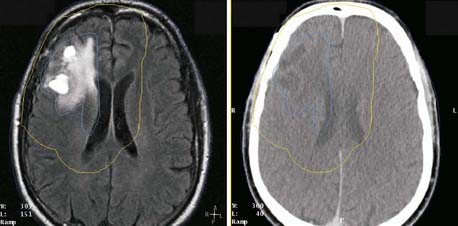
Several characteristics have limited the applicability of MR images alone for treatment planning. One is the lack of signal from cortical bone: Although bone location can be inferred in some disease sites, it is not possible to distinguish bone-air interfaces such as sinuses. Pixel intensities do not correlate with electron density as they do in CT; thus they cannot be used directly in dose calculations. Intensity variations occur across the images, such as those arising from falloff in sensitivity toward the ends of the receiver coil. The images may be distorted by variations in local magnetic fields caused by imperfections in the machine itself and by the presence of metal objects in the environment and within the patient. Because of these limitations, MR images are usually registered to CT, and the information MRI provides is transferred to the CT study for treatment planning purposes. The registration of CT and MR images is described in another section. MRI has been effectively used for treatment planning of brain, head and neck, liver, pelvis, and prostate tumors (Fig. 8-6). Other disadvantages include the high cost of equipment and site preparation, examination times that are 1.5 to 2 times those required for CT, a limited diameter of the patient tunnel opening in the magnet, and magnetic and radio-frequency shielding problems.
MRI alone can be used in treatment planning of sites that do not have large tissue inhomogeneities by substituting a bulk density correction for dose calculation in the absence of an electron density map.11
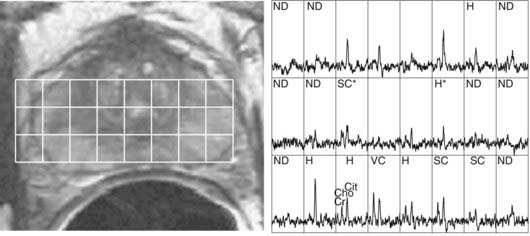
MRSI of 1H nuclei in combination with MRI is receiving increased clinical adoption for evaluating tumor extent and necrosis in brain tumors and potential staging and evaluation of prostate cancer, and thus as an aid to external beam and brachytherapy planning.12,13 The relative concentrations of metabolites choline, creatine, and citrate, specifically elevated values of the ratio (choline + creatine)/citrate, have been hypothesized to be an indicator of malignant prostatic tissue (Fig. 8-7).14,15
MRI studies of perfusion, diffusion, and contrast enhancement in recent years have shown potential for assessment of therapeutic response and as an early predictor of treatment outcome.16 These investigations indicate a potential use of MRI as an aid to optimizing therapy for individual patients.
An advantage of MRI is that rapid-sequence images can be acquired without the radiation dose associated with CT. Applications of cine MRI to radiotherapy have evaluated the influence of rectal and bladder filling on prostate motion and respiration-induced motion of anatomic structures in the thorax.17,18
Recent Developments
There is also a new class of MRI contrast agents, which are capable of gene transfection into cells. These agents will allow gene therapy to be monitored by MRI through cotransported MRI contrast agents. Additional refinements include MRI contrast agents that will enable the MRI signal to be greatly amplified through association of the site-directed contrast agent with its target.19
Ultrasound Imaging
Uses and Limitations in Radiation Oncology
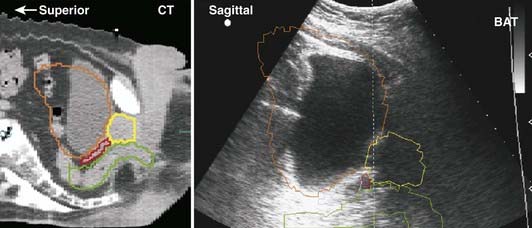
Early medical ultrasound B-scan units provided some of the first cross-sectional images for treatment planning; however, current CT and MRI units, although 5 to 10 times more expensive, produce images with better resolution and contrast that are more desirable for treatment planning. The major exception is the use of high-resolution images of the prostate obtained with an ultrasound imager that employs a special rectal probe. Transrectal ultrasound has become the predominant image modality for planning and delivery of prostate implants using transperineal template-guided insertion. Intraoperative ultrasound has also been applied to evaluate high-dose-rate prostate brachytherapy implants.20 Application of ultrasound in breast cancer occurs most commonly in the initial diagnostic evaluation: in addition to detecting abnormalities, it is useful in assessing the presence of multicentric tumor.21 Ultrasound can serve to define involved primary and nodal sites for breast-cancer radiotherapy and postlumpectomy radiotherapy. A commercially available device allows ultrasound-guided patient positioning for external-beam radiation therapy; several centers have reported on its application to daily localization and correction of prostate position.22
Ultrasound beams cannot penetrate gas-filled cavities or bone. This limits the use of ultrasonography. Furthermore, ultrasonographic images are much more difficult to interpret than those from CT or MRI (Fig. 8-8).
Recent Developments
Imaging systems consisting of multielement transducers can produce ultrasonographic images in a slice at rates fast enough to show smooth motion on a video screen (≈30 pictures per second). All modern ultrasonographic units use a digital scan converter to produce digital images. Individual pictures, or frames, can be transferred to PACs or to film with a laser imager. Recent ultrasound-guided patient positioning systems produce 3-D ultrasound images, thereby improving image interpretation and alignment relative to earlier 2-D systems.23
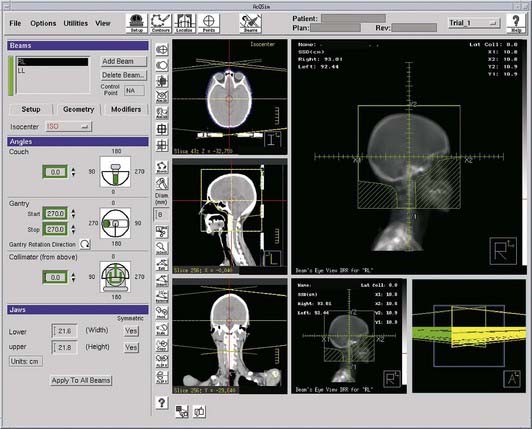
Computed Tomography Simulation
 Target and normal organ localization by defining contours on axial CT images, or by virtual fluoroscopy.
Target and normal organ localization by defining contours on axial CT images, or by virtual fluoroscopy. Virtual fluoroscopy, which allows definition of a treatment portal without requiring contour segmentation of the target on all axial CT slices. The computer displays AP and lateral DRRs, and mimics the movement of a conventional fluoroscopic simulator in the vertical, transverse, and longitudinal directions, and symmetric or asymmetric collimation as well as collimator rotation.
Virtual fluoroscopy, which allows definition of a treatment portal without requiring contour segmentation of the target on all axial CT slices. The computer displays AP and lateral DRRs, and mimics the movement of a conventional fluoroscopic simulator in the vertical, transverse, and longitudinal directions, and symmetric or asymmetric collimation as well as collimator rotation. Definition of reference isocenter points directly from the volumetric CT data, followed by CT table positioning such that alignment lasers indicate triangulation points for marking on the patient surface.
Definition of reference isocenter points directly from the volumetric CT data, followed by CT table positioning such that alignment lasers indicate triangulation points for marking on the patient surface. Generation of DRRs for comparison with portal films or electronic portal images. The DRR display may include a treatment field outline, graticule tray, and outlines of organs (Fig. 8-9).
Generation of DRRs for comparison with portal films or electronic portal images. The DRR display may include a treatment field outline, graticule tray, and outlines of organs (Fig. 8-9). Transfer of CT images and associated simulation data to a radiation treatment planning system for dose calculation and plan evaluation.
Transfer of CT images and associated simulation data to a radiation treatment planning system for dose calculation and plan evaluation.Imaging in Treatment Verification
Portal Radiographs
Portal radiographs are a fundamental part of the clinical quality-assurance program. They provide a beam’s eye view record of the patient treatment volume, radiographically revealing the relationship between anatomical structures and the treatment field. There are three types of imaging systems available for acquiring portal radiographs: film, electronic portal imaging devices (EPIDs), and CR; each system is described in later sections. The American Association of Physicists in Medicine (AAPM) Task Group 28 defines a portal radiograph as “a radiograph produced by exposing the image receptor to the radiation beam which emanates from the portal of a therapy unit.”24 The report describes three types of radiographs (within the context of acquisition with film):
 Localization: A portal radiograph produced by an exposure that is short compared with the daily treatment of that field (generally called localization films, beam films, or port films).
Localization: A portal radiograph produced by an exposure that is short compared with the daily treatment of that field (generally called localization films, beam films, or port films). Double exposure: A localization film composed of two sequential exposures; the first is that of the shaped treatment field, and the second of a larger rectangular field, superimposed on the first. The double-exposure portal makes it easier to relate the treatment field to the surrounding anatomy and is particularly useful for relatively small field sizes.
Double exposure: A localization film composed of two sequential exposures; the first is that of the shaped treatment field, and the second of a larger rectangular field, superimposed on the first. The double-exposure portal makes it easier to relate the treatment field to the surrounding anatomy and is particularly useful for relatively small field sizes.The importance of portal radiography as a quality-assurance tool is supported by numerous published studies, which have made it clear that portal radiographs are essential to accurate radiotherapy and that frequent imaging is desirable for difficult patient setups or highly conformal treatments.25
Portal Films
The image quality of either type of film is reduced in part by high-energy secondary electrons that exit from the patient onto the film. Portal film quality is improved with specialized radiotherapy cassettes that provide metal front screens in uniform and close contact with the film and that is thick enough to absorb the shower of secondary electrons exiting the patient. The thickness of these screens is determined by measurements of scatter-to-primary ratios for large fields.24 Thicknesses are generally 1 to 4 mm, depending on the beam energy and on the screen material (usually lead or copper). A rear metal screen is also often used to improve the overall sensitivity, or speed, of the system. The use of rear screens may cause some reduction in image resolution; however, in practice no significant degradation of image quality is observed. A second important function of the film cassette is to provide good film–screen contact.
Electronic Portal Imaging Devices
The EPID is attached to the gantry of an isocentric radiation treatment machine so that it may intercept the exiting photon beam at all gantry angles. The ideal support system must be sturdy and capable of precise repositioning, yet easily retracted or removed to facilitate patient access. First-generation commercial EPIDs have been either video-based or matrix ionization chamber-based systems.27 In video-based systems, the image is formed by the interaction of the radiation with a fluorescent screen assembly consisting of a metal (e.g., copper or tungsten) plate onto which the phosphor is deposited. The metal plate is upstream relative to the phosphor and absorbs scattered electrons emanating from the patient, as well as interacting with the photons to generate high-energy electrons that, in turn, produce the fluorescent image in the phosphor. A front-silvered mirror, placed diagonally, reflects the fluorescent light by 90 degrees into a video camera. In the matrix ionization chamber system, electrode strips on two printed circuit boards form an array of ionization chambers; the strips run horizontally on one board and vertically on the other. A 1 mm gap between the boards is filled with iso-octane liquid, which serves as an ionization medium for the x-rays. An image is obtained by switching on the high voltage to one row, collecting the currents from each of the column electrodes, and repeating the process for each of the rows. The first-generation systems, although more convenient than film, have a number of limitations. The camera-based systems are bulky and image quality is reduced because of the poor optical transfer system, and the matrix ionization chamber system requires long irradiation times and is especially sensitive to small changes in dose rate, resulting in line and band artifacts.
The current commercially available EPIDs take advantage of the large industrial development of flat-display panel technology, and provide faster acquisition and superior image quality than their predecessors. Variously referred to as active-matrix flat-panel imagers or amorphous silicon flat-panel image detectors, the array of electronic circuitry comprising the pixels is fabricated on panels of hydrogenated amorphous silicon. Each pixel comprises a light-sensitive photodiode attached to a thin-film transistor acting as a switch. The physical buildup of the detector array consists of a metal plate (typically 1 mm copper) and phosphor screen to convert x-rays to visible light; the metal layer also serves to attenuate scattered radiation. The light is converted to electron-ion pairs in the photodiode, and collected by application of a bias voltage onto a storage capacitor. The charges stored in the pixels are transferred to the readout electronics by activating the pixel switches row by row and reading out all columns simultaneously. The arrays are capable of 10 frames per second or higher, and thus are applicable to fluoroscopy as well as radiography. For portal imaging applications, several frames are averaged together to reduce image noise. Panel sizes of 30 × 40 cm2 or 40 × 40 cm2 are available in arrays of 512 × 384, 1024 × 768, or 1024 × 1024 pixels, respectively (Fig. 8-10).
Use of Portal Radiographs in Radiation Oncology
Image Registration
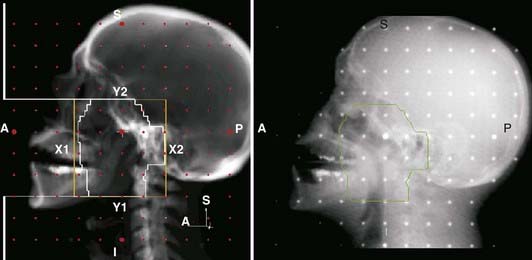
Portal radiography is a primary tool for quality assurance in radiation delivery. The portal image from the first treatment is compared with the corresponding reference image (a digitized simulator film or digital image, or digitally reconstructed radiograph from CT) to ensure correct patient setup and proper block construction and placement or MLC programming. Since portal (and simulator) radiographs of oblique fields are confusing and difficult to interpret because of their nonstandard view of radiographic anatomy, AP and lateral portal films are generally used to verify the isocenter location. Side-by-side comparison of the simulator and portal images can be facilitated with the use of a graticule, a device that projects a cross-hair shadow and centimeter scale in the portal film; the device is supplied by several vendors.24,28 The use of such a device is recommended because the cross hairs of the linear accelerator or cobalt-60 fields do not appear on the portal image. If field adjustments are needed, this tool facilitates the quantification and communication of the desired changes (see Fig. 8-10).
Two of the most common methods of interactive, or manual, image registration are the line drawing or template technique, and point-pair registration. Point-pair registration involves identifying the positions of corresponding anatomical landmarks in the reference and portal images; the portal image is then transformed (i.e., translated, rotated, and scaled) such that the selected points align with those in the reference image. Although the methodology is simple, point-pair registration is error prone, because of the difficulty in correctly identifying corresponding fiducial points, particularly when reference and portal images are of different image quality (i.e., kV vis-à-vis megavoltage beam quality). The template method involves drawing (graphically, by means of a mouse or trackball) lines or curves indicating the field edge and patient anatomy on the reference image, which is then overlaid and aligned with the portal image (Fig. 8-11). Semiautomatic extensions of the template method are those that automatically extract anatomic features in the portal image and align them with the reference template. A third category of registration methods uses automated pixel-by-pixel, grayscale intensity–based image correlation. This approach assumes similar quality images for matching; thus, it requires registration of a first-day portal image to the reference image by some other means, then uses the first-day portal image as reference for subsequent treatment sessions.
As previously indicated, portal radiographs are part of the overall quality-assurance program for ongoing verification of treatment accuracy. Portal images can reveal errors in the patient setup position, field size and orientation, or placement and shaping of shielding blocks. Thus they should be taken during the initial treatment setup and weekly thereafter, as recommended by the AAPM Task Group on Radiotherapy Portal Imaging Quality and the AAPM Task Group on Comprehensive Clinical Quality Assurance.28 Regular review takes place at the weekly chart (film) rounds; the current week’s portal films are compared side by side with the appropriate reference image. Alternatively, electronic images can be displayed by means of a computer monitor or liquid crystal display projector. Approved portal images should be signed (on the film electronically via a radiotherapy PAC) and dated for medicolegal documentation purposes, and discrepancies should be investigated and rectified. Note that altering the patient setup position or skin marks based on a small discrepancy (e.g., <0.5 cm) observed in a single portal image may not be appropriate. Rather, it may be more appropriate to take a repeat image on the subsequent day before effecting a change. However, by using online imaging with implanted markers, treatment accuracy can be improved such that changes of less than 0.5 cm can be made accurately.
Specific goals for using EPIDs should be established before they can be put to clinical use.25,27 A number of key questions should be addressed; these questions include:
 What is the primary purpose of introducing EPIDs into the clinic—as simple film replacement, improved patient positioning, or assessment of localization errors and adequacy or margins?
What is the primary purpose of introducing EPIDs into the clinic—as simple film replacement, improved patient positioning, or assessment of localization errors and adequacy or margins? For which patients and protocols will EPIDs be used—all patients, selected sites, or selected patients?
For which patients and protocols will EPIDs be used—all patients, selected sites, or selected patients? What will be the imaging frequency and type of acquisition—daily, weekly, single or double exposure, all or some fields?
What will be the imaging frequency and type of acquisition—daily, weekly, single or double exposure, all or some fields? What type of image evaluation—immediately prior to the treatment fraction or between fractions, visual or computer-based analysis?
What type of image evaluation—immediately prior to the treatment fraction or between fractions, visual or computer-based analysis? Where will image evaluation take place—at the treatment unit, in a central work area, or department-wide on any workstation?
Where will image evaluation take place—at the treatment unit, in a central work area, or department-wide on any workstation?Other Applications of Electronic Portal Imaging Devices
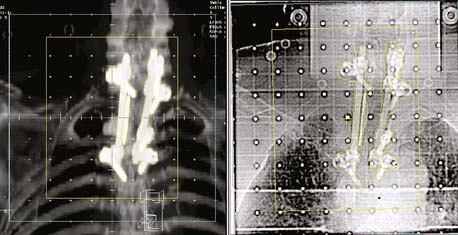
The use of EPIDs for detecting radio-opaque fiducial markers implanted in or near a tumor, particularly in the prostate, has received increasing attention as a means of correcting errors in target position from internal organ motion.26
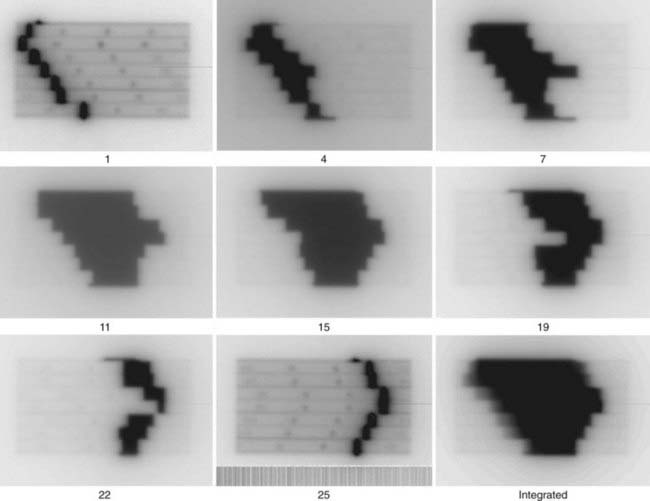
Another application is to verify intensity-modulated treatment fields delivered with an MLC. With present algorithms, this is usually accomplished by irradiation of a flat phantom before actual treatment.29 The images of the subfields composing the intensity-modulated field are summed in the computer to obtain a 2-D intensity pattern (Fig. 8-12), which is compared with the intended pattern from the treatment planning system. Other uses of EPIDs include transit dosimetry to measure the accuracy of dose delivery during treatment, and in accelerator quality-assurance procedures.30,31
Limitations
There appears to be a systematic degradation of portal image quality with increasing accelerator beam energy. This is attributable to a reduction in subject contrast and in resolution, although the relative importance of these factors is uncertain. Although the decrease in contrast with photon energy, changing from the kiloelectron volt (keV) to megaelectron volt (MeV) range, is a result of the reduced probability of photoelectric interaction, the reduction in image quality with sources higher than 1 MeV cannot be similarly explained and may be caused in part by the increased range of Compton electrons generated in the front screen for film, or in the conversion plate and phosphor screen for CR and electronic portal imaging systems.32 At the high end of megavoltage photon energies, the situation is further complicated by increased multiple photon scattering and some increase in pair production. Additional degradation of cobalt-60 beam images is caused by blurring caused by the relatively large cobalt-60 source size.
Portal Radiograph Quality Assurance
Most EPIDs require a calibration process to correct for inherent systematic artifacts (e.g., variations in sensitivity across the detecting device). For amorphous silicon EPIDs, the electronic noise and sensitivity of the photodiode pixels can vary with time. The AAPM Task Group 58 has reported on acceptance testing, commissioning, and periodic quality assurance of EPIDs.27 The recommended quality-assurance tasks include tests of the collision interlock system that prevents collision of the EPID with the couch or patient, accuracy of the EPID positioning relative to the isocenter, image artifacts, image resolution and noise using phantoms designed for this purpose, and geometric localization accuracy.
In-Room Kilovoltage Radiography
The advent of large-area flat-panel x-ray detectors has led to widespread use of kV image guidance systems for patient positioning in the treatment room. At present, several kV imaging developments have evolved into commercial products that use one or more image detectors together in combination with kV x-ray sources in the treatment room.33–37 The near-diagnostic quality of kV radiographs facilitates automatic image registration and beam alignment just prior to treatment or in real time during treatment, often in combination with implanted fiducial markers (Fig. 8-13). A single radiograph provides 2-D information, whereas two imaging systems enable stereoscopic 3-D imaging. Gantry-mounted systems (Fig. 8-14) provide radiographic as well as cone-beam CT capabilities (described in the next section); such systems are generally mounted at right angles to the treatment beam.
In-Room CT-Based Systems
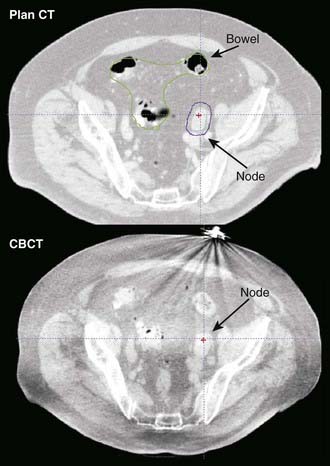
The principal advantage of CT-based systems in the treatment room over radiographs is that they provide volumetric anatomical information, including soft tissue localization. The use of repeat CT scans during treatment as a means of measuring and reducing target positioning errors has become increasingly widespread; Chapter 12 gives a more extensive description. Different types of systems for imaging the patient at the treatment accelerator are commercially available. “CT-on-rails” systems combine a linear accelerator and conventional CT scanner in the treatment room.38 Helical tomotherapy systems use a CT detector consisting of pressurized xenon gas within a tungsten septa-filled housing with the megavoltage beam (Fig. 8-15). Cone-beam CT systems make use of a 2-D flat-panel imaging sensor to acquire a volumetric image in a single rotation; such systems have been developed for use with megavoltage as well as kV x-ray sources (Fig. 8-16).39,40 The cone-beam geometry is actually pyramidal in shape, diverging both in longitudinal and lateral directions with its apex at the x-ray source. The source-detector pair moves around the patient to collect a set of 2-D projection images, which are then processed with a cone-beam reconstruction algorithm to obtain a 3-D image set.
Electromagnetic Marker Tracking
An emergent localization technology uses implanted electromagnetic transponders whose position is detectable with nonionizing radiation, providing continuous real-time monitoring. The position of the transponders is detected at a rate of 10 Hz with an electromagnetic source-detector array placed close to the patient.41 Application of this system for prostate localization and real-time position monitoring has been demonstrated.42
Optical Methods
Optical imaging systems provide positional information on the external anatomy, and commonly use infrared light to determine an object’s location. The object can be active, such as an infrared emitter; passive markers consisting of spheres or disks that reflect the infrared light; or the patient’s surface acting as a reflector. The optical detectors are usually CCD cameras, which are a collection of light-sensitive cells or pixels arranged in a 2-D array, producing a digital image. An optical system consisting of two such CCD cameras provides stereoscopic 3-D information of the detected objects (Fig. 8-17).
The first commercially available optical system for radiotherapy localized patients by means of detecting passive markers attached to a biteplate.43 An early system for extracranial treatment enabled tracking of reflective spheres attached to the patient’s torso.44 A respiratory monitoring system, in which infrared light from an illuminator is reflected from a passive reflective block placed on the patient and detected by video camera, is widely used in connection with respiration-correlated CT, breathing-synchronized fluoroscopy, and gated treatment on a linear accelerator.7 More recent optical patient positioning systems use stereoscopic imaging of a speckle pattern projected onto the patient,45 or imaging of the intersection of a scanned fan-shaped, laser beam with the patient.46
Nuclear Medicine Imaging in Radiation Oncology
The strength of nuclear medicine imaging is that it is several orders of magnitude more sensitive than other imaging modalities. How sensitive depends on the radiotracer used. For example, the typical concentration of 18F-fluorodeoxyglucose (FDG) uptake in a lesion is 0.5 picomole (i.e., almost nine orders of magnitude lower than the mmole concentrations required for 19F-FDG NMR).47 Nuclear medicine operates by the “tracer principle,” in which a biomolecule is labeled, is injected into the body, and serves as a precursor for the biomolecule it mimics. Some tracers are chemically identical to the biomolecules they mimic (e.g., 11C-glucose or 11C-methionine). Others are synthetic precursors such as 18F-FDG and 123I, 124I, or 131I-iododeoxyuridine, which are not chemically identical, but sufficiently similar to participate in the biochemical process of interest, albeit with slightly altered metabolic rates. The number of possible tracers for nuclear medicine imaging is potentially infinite.
Single Photon Emission Gamma Camera Imaging
Uses and Limitation of Single Photon Emission Gamma Camera Imaging in Radiation Oncology
Nuclear medicine studies are rarely the primary method of choice for the diagnosis of tumors, with the exception of the role of radioiodine in thyroid cancer, for which it also plays an important role in therapy.48 Nuclear medicine is a sensitive method for the detection of metastatic spread of disease, the most widely used of which is the bone scan agent technetium-99m methylene diphosphate, for determining the extent of metastatic spread to the bone.49 A methodology to quantitatively track the progression of bone metastases has been proposed by Imbriaco and colleagues,50 and is called the bone scan index. A broader class of tumor diagnostic (and therapeutic) tracers includes the somatostatin binding peptides, with selective binding to neuroendocrine cancers.51 Several groups have evaluated the use of 99mTc-sestamibi for the detection of breast cancer. One example is Lumachi and colleagues52 who reported that the sensitivity of sestamibi scintimammography reaches 100% in patients with breast lesions larger than 8 mm, and that the predictive value of mammography, sestamibi scintimammography, and mammography and sestamibi scintimammography together were 63.4%, 95.1%, and 97.6%, respectively. Lymphoscintigraphy is a procedure in which a radiotracer, 99m
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree