Brachytherapy is a fundamental component of the definitive treatment of many advanced gynecologic malignancies, most notably cancers of the uterine corpus and cervix, and allows high radiation doses to be delivered to the target while minimizing the normal tissue dose. However, dose specification has been based primarily on points visible on plain radiographs, with limited correlation to a patient’s anatomy and extent of disease. Recent advances have allowed more customized volume-based specification of dose, which has allowed improvements in outcomes. This article reviews these advances using cervical cancer as a model, and looks to future directions with this promising treatment.
Key points
- •
T2-weighted magnetic resonance imaging (MRI) gives the most specific and detailed measure of disease in the pelvis for most gynecologic malignancies.
- •
A high-risk clinical target volume (HRCTV) may be defined as residual disease on T2-weighted MRI; dose to this volume is highly correlated with control in the pelvis.
- •
High doses to even small volumes of neighboring critical tissues such as the bladder and rectum are correlated with late toxicity to these organs.
- •
Modern high-dose-rate applicator design allows optimal coverage of the HRCTV while minimizing normal tissue volumes.
- •
An international trial (the EMBRACE [European Study on MRI-guided Brachytherapy in Locally Advanced Cervical Cancer] study) is ongoing, designed to assess the exportability of these findings in the broader community.
Introduction to brachytherapy
Brachytherapy has been an integral portion of the curative treatment of several gynecologic malignancies for more than 100 years, beginning with the use of intracavitary radium for inoperable uterine cancer and cervical cancer in the early 1900s. The advantages of this form of radiation delivery compared with external beam treatment derive primarily from the inverse square law: with movement away from an implanted radioactive source, the dose decreases at the inverse square of the distance:
with D a being the dose at a point d a far from the source and D b being the dose at point d b from the source. Thus the dose at 2 cm from the source is one-quarter that of the dose at 1 cm, whereas the dose at 10 cm is one-hundredth of the same dose.
Therefore, when it is possible to place a source within the target, very high doses are achievable centrally, with a sharp decrease of dose at the target boundary. In addition, because the radioactive sources are placed directly within the tumor, the risk of the target migrating out of the field (or conversely, normal tissue migrating into the field) is essentially eliminated.
Film-based treatment planning
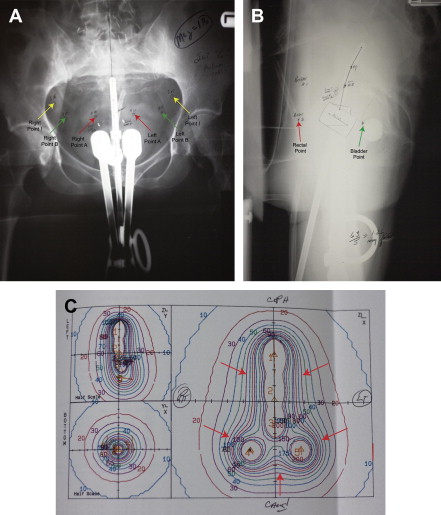
The methods of treatment planning for tandem and ovoid-based brachytherapy for cervical cancer remained unchanged throughout the later portion of the twentieth century. The Manchester, Stockholm, and Paris methods of dose specification and loading patterns for low-dose-rate (LDR) implants were developed in 1927 to 1938, later refined and modernized by Fletcher and colleagues at MD Anderson in the late 1940s. These systems were based on imaging technology available at the time of their initial conception and development, namely orthogonal plain films of the pelvis with the implant in place. By necessity, surrogates visible on plain radiographs were used for estimating doses to both the target and to the critical normal tissue. Point A, or the paracentral dose, defined at a point 2 cm superior to the top of the ovoids, and 2 cm lateral to the tandem, evolved into a prescription point in these systems ( Fig. 1 ).