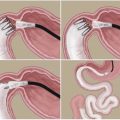
Fig. 8.1
Ileal transposition surgery. (a) Normal intestinal tract showing the location of transections of the ileum and duodenum. (b) A segment of the transected ileum is transposed in the same direction just distal to the duodenum
Weight loss following IT surgery is often associated with a reduction in fat mass (Kohli et al. 2010; Koopmans et al. 1982), with minimal or no malabsorption (Chen et al. 1990; Strader et al. 2005). We demonstrated that IT surgery leads to greater weight loss than pair-fed animals, suggesting that increased energy expenditure may contribute to weight loss (Chelikani et al. 2010). Others have shown that IT stimulates energy expenditure in rats (Cummings et al. 2013; Kohli et al. 2010). However, the relative contributions of reductions in food intake and increased energy expenditure to the reduction in body weight following IT surgery remain to be determined.
8.3 Ileal Transposition Surgery: Effects on Diabetes Control
In contrast to the abundance of literature on the hypophagic and weight loss effects, there is minimal information on the effects of IT surgery on diabetic control. In earlier studies with diet-induced obese rats, IT was shown to improve insulin sensitivity but not glucose tolerance (Strader et al. 2005). In Goto–kakizaki diabetic rats, improvements in glucose tolerance were observed within a month following IT surgery (Chen et al. 2011; Kindel et al. 2009; Patriti et al. 2004, 2005, 2007; Wang et al. 2008; Yan et al. 2012), but either with (Wang et al. 2008) or without accompanying improvements in insulin sensitivity or insulin secretion (Patriti et al. 2004; Patriti et al. 2005, 2007), suggesting that insulin-independent mechanisms could also play a role in the glycemic improvements. Interestingly, in streptozotocin-treated diabetic, and normal euglycemic rats (Strader et al. 2009), diet-induced obese rats (Kohli et al. 2010), overweight rats (Mencarelli et al. 2013; Nausheen et al. 2013; Ramzy et al. 2014), obese and diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats (Ikezawa et al. 2012), University of California at Davis type 2 diabetes mellitus (UCD-T2DM) rats (Cummings et al. 2010, 2013), and the fatty Zucker rats (Culnan et al. 2010), IT improved glucose tolerance and insulin sensitivity, independent of weight changes. Thus, across multiple animal models of diabetes and obesity, IT surgery often leads to profound improvements in glycemic control and insulin sensitivity that are not dependent on weight loss. The underlying mechanisms by which IT improves diabetic control are just being unraveled.
8.4 Ileal Transposition Surgery: Mechanisms of Weight Loss and Diabetic Control
8.4.1 Gut Adaptation
The weight loss and metabolic effects of IT are often ascribed to early stimulation of the translocated lower gut by ingested food. Ileal transposition produces robust structural and functional adaptations in the transposed segment. Some of these include increased villus height and width, increased mitogenic capacity, increased muscle thickness, and increased weight suggestive of hypertrophic and hyperplastic changes in the transposed segment (Koopmans et al. 1984; Kohli et al. 2010; Nausheen et al. 2013; Ramzy et al. 2014). In additional to these structural adaptations, the transposed segment also adapts functionally with increased sucrase activity (Ulshen and Herbst 1985) and glucose uptake in the ileum (Culnan et al. 2010; Menge et al. 1978).
8.4.2 Role of Gut Peptides
As expected from a surgical intervention that causes early stimulation of the lower gut, IT leads to increased expression of multiple gut peptides in the transposed ileum, including glucagon-like peptide-1 (GLP-1), peptide YY (PYY) (Kohli et al. 2010; Nausheen et al. 2013; Ramzy et al. 2014; Strader et al. 2005), gastrin (Aiken et al. 1994), cholecystokinin, serotonin, and neurotensin (Hansen et al. 2014) (Fig. 8.2). A consistent finding across studies is that IT leads to increase in plasma concentrations of GLP-1 and PYY (Chelikani et al. 2010; Culnan et al. 2010; Cummings et al. 2010, 2013; Gaitonde et al. 2012; Grueneberger et al. 2013; Ikezawa et al. 2012; Kohli et al. 2010; Koopmans et al. 1984; Patriti et al. 2007; Ramzy et al. 2014; Strader et al. 2005, 2009).
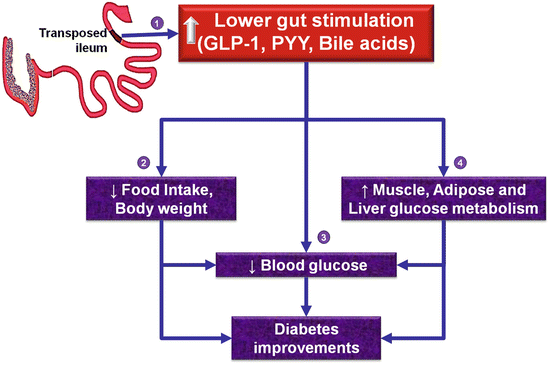

Fig. 8.2
Hypothetical mechanisms of weight loss and diabetes improvements following ileal transposition surgery. Enhanced lower gut stimulation leads to increased circulating concentrations of lower gut peptides (glucagon-like peptide-1, peptide YY) and bile acids (1) which in turn may lead to a reduction of food intake and body weight (2) with consequent indirect or direct improvements in glycemic control (3). Further, lower gut signals may improve glucose metabolism in muscle, adipose, and liver (4) with consequent diabetic improvements. However, a causative role for lower gut peptides and bile acids to the weight loss and diabetic improvements of IT surgery remains to be established
Though the increased secretion of these L-cell products likely mediates the “ileal brake” phenomenon, and decreases proximal gut motility (Ohtani et al. 1999), it is unknown whether the decreased motility contributes to the hypophagic effects of IT surgery. The association of increased plasma concentrations of the incretin—GLP-1, with improvements in glucose tolerance, suggests that GLP-1 may play a role in the glycemic improvements of IT (Culnan et al. 2010; Cummings et al. 2010, 2013; Gaitonde et al. 2012; Ikezawa et al. 2012; Johannessen et al. 2013; Kohli et al. 2010; Koopmans et al. 1984; Nausheen et al. 2013; Patriti et al. 2004, 2007; Ramzy et al. 2014; Strader et al. 2005, 2009; Wang et al. 2008). Systemic administration of the GLP-1 receptor antagonist exendin (9–39) has been shown to attenuate the improvements in glucose tolerance following IT, providing strong evidence for a direct role for GLP-1 receptor signaling, in the glycemic improvements of IT surgery (Gaitonde et al. 2012). However, it remains, to be determined whether GLP-1 and other gut peptides mediate the effects of IT surgery on food intake and body weight, and whether other gut peptides that are upregulated also directly mediate the effects of IT surgery on glycemic improvements. Though Dr. Koopmans noted that the weight of the pancreas is increased with IT surgery (Koopmans et al. 1984), the effects of IT surgeries on insulin concentrations were inconsistent with some studies, reporting either no change (Grueneberger et al. 2013; Ramzy et al. 2014; Strader et al. 2009), decrease in rats (Culnan et al. 2010; Cummings et al. 2010; Ikezawa et al. 2012; Koopmans et al. 1984; Nausheen et al. 2013), or increase (Cummings et al. 2013); these discrepancies could likely be due to differences in the experimental model, length of the transposed segment, frequency of sampling, and/or duration of follow-up after surgery. In general, IT surgery decreases plasma leptin concentrations, reflective of reductions in adipose reserves (Gaitonde et al. 2012; Ikezawa et al. 2012; Kohli et al. 2010; Nausheen et al. 2013; Ramzy et al. 2014). Despite changes in circulating gut hormone concentrations, it is still unknown whether these peptides act via paracrine, neurocrine, and/or endocrine routes to mediate the effects of IT surgery on energy balance and glucose homeostasis. Further, the relative importance of the enteric and central neural networks, in the metabolic effects of IT surgery, also remains to be determined (Fig. 8.2).
8.4.3 Enterohepatic Bile Acid Cycling
Apart from a role for gut peptides in the improvements in glycemic control, less is known of the downstream molecular events by which IT surgery improves glucose and lipid metabolism in peripheral tissues. Given that ileum is the major site for bile acid reabsorption, that bile acids play an important role in lipid metabolism, and that circulating bile acids and fibroblast growth factors are often increased following RYGB together with diabetic improvements in humans (Gerhard et al. 2013; Simonen et al. 2012), the mechanisms by which IT alters bile acid metabolism are just being characterized. Circulating concentrations of total, primary, conjugated, and non-conjugated bile acids have been shown to be increased in rats following IT surgery (Cummings et al. 2010, 2013; Kohli et al. 2010; Mencarelli et al. 2013). These changes are associated with an upregulation of the molecules that stimulate bile acid uptake in the intestine (e.g., ASBT, GPBAR1) and a downregulation of transcripts of key molecules involved in bile acid synthesis in the liver (e.g., Cyp7A1, Cyp8B1, Cyp7B1, Cyp27A1) (Cummings et al. 2013; Kohli et al. 2010). These studies are correlative and suggest that by modulating enterohepatic bile cycling, IT surgery likely contributes to improvements in glucose homeostasis (Fig. 8.2); however, it is unknown whether these alterations in bile cycling directly mediate the hypophagic and weight loss effects.
8.4.4 Glucose Metabolism in Skeletal Muscle, Adipose, and Hepatic Tissues
Skeletal muscle, liver, and adipose tissues account for about 80 %, 5 %, and 1 % of total glucose metabolized, respectively, in humans (DeFronzo et al. 1981). Excess lipid deposition in muscle and liver is a key contributing factor to impaired insulin signaling and diabetes (Samuel and Shulman 2012). We provided further insights into the role of muscle and adipose to the metabolic benefits from IT and demonstrated that the transcript and protein abundance of GLUT-4, the insulin-sensitive glucose transporter, is increased in muscle and adipose tissues following IT which is suggestive of enhanced glucose clearance by these tissues (Fig. 8.2) (Nausheen et al. 2013; Pezeshki and Chelikani 2014; Ramzy et al. 2014). Though IT did not affect expression of GLP-1 receptor in muscle, it increased the protein abundance of IRS-1 whereas IRS-1 pS636, a negative modulator of IRS-1, was decreased suggestive of enhanced insulin sensitivity (Nausheen et al. 2013; Pezeshki and Chelikani 2014; Ramzy et al. 2014). We also demonstrated that IT increased the protein abundance of muscle AMPKα (a key regulator of nutrient metabolism), increased transcript abundance of the rate-limiting glycolytic enzymes (hexokinase and phosphofructokinase) in muscle, adipose, and liver tissues suggestive of enhanced glycolytic flux, and increased mitochondrial lipid oxidative markers (CPT-1, MCAD, and COX-IV) in muscle while decreasing lipogenic transcripts (FAS) in fat and liver suggestive of decreased lipogenic and enhanced lipid oxidative capacities (Nausheen et al. 2013; Pezeshki and Chelikani 2014; Ramzy et al. 2014). However, the relative importance of gut peptides, bile acids, and neural mechanisms in mediating the observed metabolic effects of IT in peripheral tissues are still unknown (Fig. 8.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree