© Springer Science+Business Media New York 2016
Marina Kurian, Bruce M. Wolfe and Sayeed Ikramuddin (eds.)Metabolic Syndrome and Diabetes10.1007/978-1-4939-3220-7_1515. Ileal Interposition with Sleeve Gastrectomy for the Treatment of Type 2 Diabetes
(1)
Hospital de Especialidades, Sao Paulo, Brazil
Keywords
Ileal InterpositionSleeve gastrectomyType 2 diabetes mellitusObesity15.1 Introduction
Type 2 diabetes mellitus (T2DM) and obesity are predicted to be two of the greatest public health problems of the coming decades globally. Worldwide, the prevalence of Diabetes is increasing and vascular complications are the main cause of death [1]. Indeed, during 1988–2000 the annual all-cause mortality rates among T2DM patients were 25.2 per 1000 person-years compared with 9.5 per 1000 person-years in those without diabetes in the US population age 35–74 years. Cardiovascular (CV) disease mortality in this diabetic population was 11.1 per 1000 person-years compared to 3.4 per 1000 person-years in those without diabetes [2].
The possibility that gastrointestinal surgeries may lead to improvement in glucose homeostasis through mechanisms beyond reduced food intake and weight loss have been extensively explored. The different bariatric procedures can be mainly restrictive, malabsorptive, and mixed. As there is not an ideal operation, a number of variations of each of these procedures have been performed over the years, in order to optimize the results and decrease their disadvantages. Our incomplete understanding of the physiology of normal appetite and satiety regulation, and the pathophysiology of obesity, are certainly key points in explaining the multiple surgical alternatives. A quite recent meta-analysis supports the assumption that the most effective operations in relation to weight loss and resolution of associated diseases are the biliopancreatic diversion (BPD) and duodenal switch (DS) . However, the overall morbidity is high and there is an increased risk of significant malabsorption with an attendant requirement for indefinite supplementation [3].
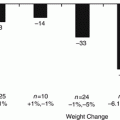
An analysis of 621 studies in the literature, including over 135,000 morbidly obese patients undergoing bariatric surgery, reported resolution of T2DM in 78 % of cases [4]. Furthermore, in a retrospective cohort of 7925 bariatric patients, deaths attributed to diabetes were reduced by a remarkable 92 % [5]. Thus, in the morbid obese patient with T2DM, bariatric surgery appears to be a highly effective treatment alternative. However, when using a more restricted definition of diabetes resolution, with complete remission defined as glycated hemoglobin (HbA1c) less than 6 % and fasting blood glucose (FBG) less than 100 mg/dL off diabetic medications, Brethauer et al. [6] demonstrated long-term (5 years or more) complete remission of T2DM in 31 % of patients following gastric bypass, 9 % after sleeve gastrectomy and none after gastric banding.
On the other hand, the most frequent kind of T2DM, the hyperglycemia after the fourth decade of life in moderately obese subjects, is a progressive disease, and resolution, whether spontaneous or by treatment, is uncommon [7]. Saydah SH et al. [8] demonstrated that only 7 % of 404 adult diabetic patients from the NHANES study achieved HbA1c < 7 %, blood pressure <130/80 mmHg and total cholesterol < 200 mg/dL %. From the Steno2 study [9], approximately 17 % of the patients were able to reach HbA1c below 6.5 %. A strategy of intensive glucose control to lower the HbA1c value to 6.5 % yielded a 10 % relative reduction in major macrovascular and microvascular events [10]. In another study, an intensive glucose control in patients with poorly controlled T2DM had no significant effect on the rates of major CV events, deaths, or microvascular complications [11]. In the ACCORD study [12], high risk T2DM patients submitted to intensive therapy to lower HbA1c had an increased mortality and no significantly reduced major CV events, as compared with standard therapy. So, there is a clear need to offer diabetic patients an alternative treatment modality with better results.
A straightforward option would be to push the limits of the indication of the different bariatric surgeries to non-morbid obese diabetic patients. However, it is not easy to justify some of the tactic components of the different bariatric operations, like the small stomach performed with the gastric bypass and the important malabsorption associated to the BPD-DS surgeries. Recent data have demonstrated that the good results of bariatric surgeries to morbid obese diabetic patients could not be reproduced for lower body mass index (BMI) patients. Schauer et al. [13] conducted a prospective, randomized, controlled trial in 150 obese patients with uncontrolled T2DM to receive either intensive medical therapy alone or intensive medical therapy plus Roux-en-Y gastric bypass or sleeve gastrectomy. Mean BMI was 36. The primary end point was HbA1c level of 6.0 % or less. At 36 months, 38 % of patients following gastric bypass, 24 % after sleeve gastrectomy and 5 % in the intensive medical treatment were able to reach HbA1c ≤ 6. In another randomized trial at four teaching hospitals, Ikramuddin et al. [14] compared lifestyle-intensive medical management and Roux-en-Y gastric bypass surgery. The primary outcome was considered successful if patients achieved the composite of a triple end point: an HbA1c of less than 7.0 %, an LDL cholesterol level of less than 100 mg/dL and systolic blood pressure less than 130 mmHg. After 12 months, 49 % of the patients in the gastric bypass group and 19 % in the lifestyle-medical management group achieved the primary end points.
An alternative to the different bariatric surgeries would be an operation, specifically designed to the treatment of T2DM, and based on the pathophysiology of the disease; very much like a regular antidiabetic medication.
According to the literature, ileal interposition was first described in 1928 [15]. Dorton in 1985 and Halberg in 1986 [16, 17] first suggested its use for the treatment of obesity. Dr. E. Mason, in 1999 [18], suggested its use for both obesity and/or diabetes. In 2003, we proposed the combination of an ileal interposition up into the jejunum (JII-SG) or into the duodenum (DII-SG) associated to a tailored sleeve gastrectomy [19]. Further on, a selected group of 19 morbid obese patients had ileal interposition associated with a sleeve gastrectomy. In this highly selected group, diabetes was resolved early in the postoperative period [20].
15.2 Pathophysiology of T2DM and Ileal Interposition with Sleeve Gastrectomy
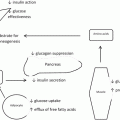
Under normal physiological conditions , unabsorbed nutrients can achieve the distal small intestine (ileum), resulting in the activation of a neuroendocrine negative feedback mechanism, the “ileal brake.” These combined effects influence digestive process, ingestive behavior, glucose and lipid metabolism and involves a number of different mechanisms, including increased secretion of peptides, like glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) from L cells of the ileum [21]. The relevance of the ileal brake as a potential target for weight and metabolic management is based on several findings: First, activation of the ileal brake has been shown to reduce food intake and increase satiety levels. Second, activation of the ileal brake determines weight loss and improves glycemic control. Third, these effects seem to be maintained over time [22].
The pathophysiology of T2DM involves failure of beta-cells to secrete adequate amounts of insulin, insulin resistance (IR) in peripheral tissues and liver, increased endogenous glucose production, accelerated lipolysis, deficiency or incretin resistance, hyperglucagonemia, increased glucose reabsorption in the kidneys, and insulin resistance in the brain [23]. The complexity of the pathophysiology is such, that the whole is bigger than the sum of the parts. Furthermore, there are other key concepts to be evaluated: (1) The pathophysiology of T2DM has also genetic and environmental components; (2) The different hormones, peptides and other agents are part of a complex regulatory system, with emphasis that multiple redundant and compensatory factors exists; (3) There is no animal model that matches the complex etiology of human T2DM; (4) During the progression of the disease, diabetic patients have different responses to the therapy applied, a greater chance of hypoglycemia and they are prone to gain weight; (5) An individual approach is suggested, with multiple targets (glycemic control, dyslipidemia normalization, blood pressure stabilization, microalbuminuria reversion, adjustable and long-lasting weight control).
Although the cellular mechanisms underlying ileal interposition with sleeve gastrectomy remains speculative, the operation intends to primarily target the pathophysiology of T2DM. The first characteristic of the operation is to provide an early contact of ingested nutrients to the interposed distal ileum resulting in an early and significant rise of glucagon-like peptide 1 (GLP-1), with its consequent impact on the defective early (first-phase) insulin secretion. The second characteristic is the correction of the defective amplification of the late phase plasma insulin response to glucose by GIP. Both characteristics were addressed in a publication of the hormonal changes before and after ileal interposition with sleeve gastrectomy [24]. The third characteristic is the amelioration of insulin resistance. An attractive hypothesis for the rapid improvement of insulin sensitivity and associated pancreatic beta-cell function could be related to short-circuiting the entero-hepatic bile acid recycling through an early reabsorption of primary bile acids. Another possibility could be related to surgical ablation of the majority of GIP-secreting intestinal K-cells. The association of variable amounts of stomach resection, tailored to weight, in a sleeve format intends to provide long-lasting control of obesity, to decrease caloric intake, to accelerate gastric emptying and to decrease the circulating levels of ghrelin. Based on the above pathophysiology, we assumed the possibility that the DII-SG would give better results in relation to JII-SG, as it addresses more aspects of the pathophysiology of the disease. These operations encompassed both the hindgut and foregut hypothesis.
All therapeutic procedures need to have efficacy balanced against risk. As with obesity, TD2M remains a major cause of illness and death. Although bariatric surgery appears to be the only procedure that determines a significant and long-lasting treatment for diabetic morbid obesity patients, T2DM in patients with a lower BMI can be treated with medications. It is really not the same disease, nor the same patient.
15.3 Animal Studies
Animal studies have shown that ileal interposition surgery delays the onset of diabetes in University of California at Davis type 2 diabetes mellitus rats (UCD-T2DM). This effect may be related to increased nutrient-stimulated secretion of GLP-17–36 and PYY and improvements of insulin sensitivity, beta-cell function, and lipid metabolism [25].
Patriti et al. [26] demonstrated in a nonobese type 2 diabetes rat model (Goto–Kakizaki) that ileal interposition improved glucose tolerance and was associated with a higher GLP-1 levels. The same author also demonstrated that ileal interposition improves glucose metabolism and beta-cell function through an enhanced proglucagon gene expression and L-cell number [27].
Ileal interposition was compared to different operations in animal models. Boza et al. [28] observed that obese diabetic rats when submitted to an ileal interposition with sleeve gastrectomy had a significant weight loss and diabetes improvement. The operation proved to be as effective as gastric bypass in the short term on weight progression, with no bypass of the proximal gut. In a nonobese rat model with T2DM, ileal interposition determined similar control of diabetes as BPD, with a better postoperative recovery [29].
15.4 Technique
Two different techniques have been performed: ileal interposition up into the jejunum associated with a tailored sleeve gastrectomy and ileal interposition up into the duodenum associated with a tailored sleeve gastrectomy. A standard five- to six-port laparoscopic technique is used after establishment of pneumoperitoneum.
The first technique, JII-SG, starts with division of the jejunum 20 cm from the ligament of Treitz using a linear stapler. An ileal segment of 150–170 cm is created 30 cm proximal to the ileocecal valve, interposing it peristaltically into the proximal jejunum. All three anastomoses are performed functionally side by side using 45-mm linear staplers, with care taken to close mesenteric defects with interrupted 3-0 polypropylene sutures. For standardization purposes, intestinal measurements are performed with traction along the antimesenteric border using a 10-cm marked atraumatic grasper. The tailored sleeve gastrectomy is performed according to preoperative BMI. It starts with devascularization of the greater curvature, beginning in the distal portion of the antrum, 5 cm proximal to the pylorus, or opposite to the incisura angularis or even 3 cm proximal to this point, using the Ultrasonic Scalpel or Ligasure. A 33-Fr Fouchet orogastric calibration tube is placed by the anesthesiologist along the lesser curvature toward the pylorus. The gastric resection is performed starting at the antrum or up in the body and continuing up to the angle of His using a linear 45- or 60-mm stapler. A 3-0 polypropylene running invaginating suture covers the staple line.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree