B-cell receptor (BCR) signaling is essential for chronic lymphocytic leukemia (CLL) cell survival. Many kinases in the BCR signaling pathway are being studied as potential therapeutic targets. Ibrutinib (PCI-32765) is a novel first-in-class selective inhibitor of Bruton tyrosine kinase. Preclinical evidence suggests that ibrutinib inhibits CLL cell survival and proliferation and affects CLL cell migration and homing. Early clinical data in patients with CLL and non-Hodgkin lymphoma is encouraging. It is likely that ibrutinib and other drugs targeting the BCR pathway will become an integral component of CLL therapy.
Key points
- •
B-cell receptor (BCR) signaling plays a crucial role in pathogenesis of chronic lymphocytic leukemia (CLL).
- •
Many kinases in the BCR signaling pathway are being explored as therapeutic targets such as Src family kinases, spleen tyrosine kinase, phosphoinositide 3 kinase, and Bruton tyrosine kinase (BTK).
- •
Ibrutinib (PCI-32765) is a selective, irreversible, and oral inhibitor of BTK.
- •
Preclinical data suggest that ibrutinib affects both CLL cell survival/proliferation as well as CLL cell migration/homing.
- •
Preliminary clinical data in patients with CLL is encouraging, with a 67% response rate in the 420-mg dose cohort in the relapsed/refractory CLL setting.
- •
Combination of ibrutinib with monoclonal antibodies and chemoimmunotherapy has also shown favorable early results.
- •
BCR inhibitors will likely become an important component of CLL therapeutics in the near future.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the Western world, with approximately 16,060 men and women expected to be diagnosed with CLL in year 2012 in the United States. Most patients with CLL do not need treatment at diagnosis; however, most patients need CLL-directed therapy in their life time. Chemoimmunotherapy is the current standard of care for patients with CLL needing treatment. One of the commonly used regimens is FCR (fludarabine, cyclophosphamide, rituximab). Tam and colleagues reported long-term follow-up of the FCR regimen for frontline treatment of CLL with a complete remission (CR) rate of 72% and median progression-free survival (PFS) of 80 months. Despite these impressive results, certain subgroups of patients treated with chemoimmunotherapy have less than optimal outcomes. These patients include older adults (>65 years old), those with poor-risk cytogenetics (del[17p], del[11q]), and patients with relapsed/refractory disease. Many approaches have been undertaken to improve the outcome of patients with CLL, including incorporation of drugs such as lenalidomide, ofatumumab, alemtuzumab, and bendamustine. These treatment strategies offer options after relapse after chemoimmunotherapy, but outcomes are still less than satisfactory, with approximately 4500 patients expected to die from CLL in the United States in the year 2012. This situation underscores the need to develop better therapeutics for patients with CLL.
B-cell receptor (BCR) activation signaling plays a crucial role in the pathogenesis in CLL. The BCR signaling pathway consists of immunoglobulin bound to the cell membrane, which attaches to a heterodimer consisting of CD79a and CD79b. Binding of a ligand to the membrane immunoglobulin leads to recruitment and phosphorylation of spleen tyrosine kinase (SYK) and Src family kinases (LYN), which in turn recruit and phosphorylate many kinases and adapter proteins, including Bruton tyrosine kinase (BTK). BTK is a nonreceptor tyrosine kinase of the Tec kinase family and plays a crucial role in BCR signaling. BTK is expressed in non–T-cell hematopoietic cell lineages. The BTK gene is located on chromosome Xq21.33-q22, and mutations in this gene result in X-linked agammaglobulinemia, a condition characterized by marked reduction in mature B cells, severe hypogammaglobulinemia, and increased susceptibility to infections. BTK activates downstream molecules such as nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal regulated kinase (ERK), which are involved in many cellular processes, including proliferation, survival, differentiation, apoptosis, and metabolism. Gene expression profiling has shown that BCR signaling is the most expressed signaling pathway in patients with CLL. BCR signaling is enhanced in patients with poor prognostic markers such as ZAP-70 overexpression and those with unmutated immunoglobulin heavy chain gene ( IGHV ) rearrangement. Activated BCR signaling has also been shown to be required for cell survival in the activated B-cell subtype of diffuse large B-cell lymphoma (DLBCL). Thus, multiple lines of data point to the crucial role of BCR signaling in CLL and other B-cell lymphoid malignancies. Many kinases in the BCR signaling pathway are being pursued as therapeutic targets in CLL, including LYN, SYK, phosphoinositide 3 kinase (PI3K), and BTK.
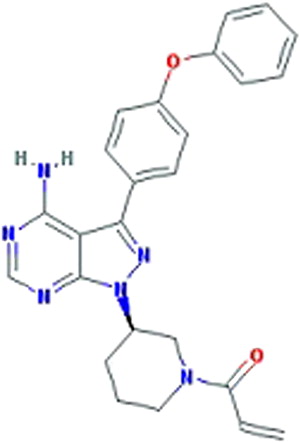
Ibrutinib (formerly PCI-32765, Pharmacyclics, Sunnyvale, CA) ( Fig. 1 ) is an oral, selective, and irreversible inhibitor of BTK and is the focus of this article. Ibrutinib was initially developed by Celera Genomics (now Quest Diagnostics, Madison, NJ) and acquired by Pharmacyclics in 2006. Ibrutinib forms a specific bond with the cysteine-481 of BTK. It leads to highly potent BTK inhibition with an IC 50 (half maximal inhibitory concentration) of 0.5 nM ( Table 1 ). Ibrutinib is orally administered with daily dosing and has no cytotoxic effect on T cells.
| Kinase | IC 50 (nM) | Fold Selectivity for BTK Inhibition |
|---|---|---|
| BTK | 0.5 | — |
| BLK | 0.5 | 1 |
| BMX | 0.8 | 1.6 |
| CSK | 2.3 | 4.6 |
| FGR | 2.3 | 4.6 |
| EGFR | 5.6 | 11.2 |
| ErbB2 | 9.4 | 18.8 |
| ITK | 10.7 | 21.4 |
| JAK3 | 16.1 | 32.2 |
| RET | 36.5 | 73 |
| FLT3 | 73 | 146 |
| TEC | 78 | 156 |
| ABL | 86 | 172 |
| c-SRC | 171 | 342 |
| LYN | 200 | 400 |
| PDGFRα | 718 | 1436 |
| JAK1 | >10,000 | >10,000 |
| JAK2 | >10,000 | >10,000 |
| PI3K | >10,000 | >10,000 |
| PLK1 | >10,000 | >10,000 |
| SYK | >10,000 | >10,000 |
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the Western world, with approximately 16,060 men and women expected to be diagnosed with CLL in year 2012 in the United States. Most patients with CLL do not need treatment at diagnosis; however, most patients need CLL-directed therapy in their life time. Chemoimmunotherapy is the current standard of care for patients with CLL needing treatment. One of the commonly used regimens is FCR (fludarabine, cyclophosphamide, rituximab). Tam and colleagues reported long-term follow-up of the FCR regimen for frontline treatment of CLL with a complete remission (CR) rate of 72% and median progression-free survival (PFS) of 80 months. Despite these impressive results, certain subgroups of patients treated with chemoimmunotherapy have less than optimal outcomes. These patients include older adults (>65 years old), those with poor-risk cytogenetics (del[17p], del[11q]), and patients with relapsed/refractory disease. Many approaches have been undertaken to improve the outcome of patients with CLL, including incorporation of drugs such as lenalidomide, ofatumumab, alemtuzumab, and bendamustine. These treatment strategies offer options after relapse after chemoimmunotherapy, but outcomes are still less than satisfactory, with approximately 4500 patients expected to die from CLL in the United States in the year 2012. This situation underscores the need to develop better therapeutics for patients with CLL.
B-cell receptor (BCR) activation signaling plays a crucial role in the pathogenesis in CLL. The BCR signaling pathway consists of immunoglobulin bound to the cell membrane, which attaches to a heterodimer consisting of CD79a and CD79b. Binding of a ligand to the membrane immunoglobulin leads to recruitment and phosphorylation of spleen tyrosine kinase (SYK) and Src family kinases (LYN), which in turn recruit and phosphorylate many kinases and adapter proteins, including Bruton tyrosine kinase (BTK). BTK is a nonreceptor tyrosine kinase of the Tec kinase family and plays a crucial role in BCR signaling. BTK is expressed in non–T-cell hematopoietic cell lineages. The BTK gene is located on chromosome Xq21.33-q22, and mutations in this gene result in X-linked agammaglobulinemia, a condition characterized by marked reduction in mature B cells, severe hypogammaglobulinemia, and increased susceptibility to infections. BTK activates downstream molecules such as nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal regulated kinase (ERK), which are involved in many cellular processes, including proliferation, survival, differentiation, apoptosis, and metabolism. Gene expression profiling has shown that BCR signaling is the most expressed signaling pathway in patients with CLL. BCR signaling is enhanced in patients with poor prognostic markers such as ZAP-70 overexpression and those with unmutated immunoglobulin heavy chain gene ( IGHV ) rearrangement. Activated BCR signaling has also been shown to be required for cell survival in the activated B-cell subtype of diffuse large B-cell lymphoma (DLBCL). Thus, multiple lines of data point to the crucial role of BCR signaling in CLL and other B-cell lymphoid malignancies. Many kinases in the BCR signaling pathway are being pursued as therapeutic targets in CLL, including LYN, SYK, phosphoinositide 3 kinase (PI3K), and BTK.
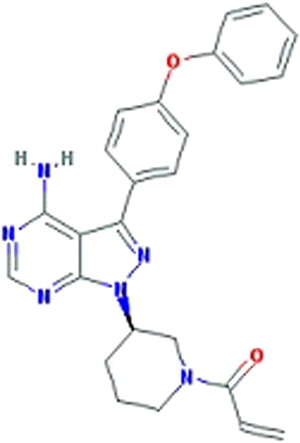
Ibrutinib (formerly PCI-32765, Pharmacyclics, Sunnyvale, CA) ( Fig. 1 ) is an oral, selective, and irreversible inhibitor of BTK and is the focus of this article. Ibrutinib was initially developed by Celera Genomics (now Quest Diagnostics, Madison, NJ) and acquired by Pharmacyclics in 2006. Ibrutinib forms a specific bond with the cysteine-481 of BTK. It leads to highly potent BTK inhibition with an IC 50 (half maximal inhibitory concentration) of 0.5 nM ( Table 1 ). Ibrutinib is orally administered with daily dosing and has no cytotoxic effect on T cells.
| Kinase | IC 50 (nM) | Fold Selectivity for BTK Inhibition |
|---|---|---|
| BTK | 0.5 | — |
| BLK | 0.5 | 1 |
| BMX | 0.8 | 1.6 |
| CSK | 2.3 | 4.6 |
| FGR | 2.3 | 4.6 |
| EGFR | 5.6 | 11.2 |
| ErbB2 | 9.4 | 18.8 |
| ITK | 10.7 | 21.4 |
| JAK3 | 16.1 | 32.2 |
| RET | 36.5 | 73 |
| FLT3 | 73 | 146 |
| TEC | 78 | 156 |
| ABL | 86 | 172 |
| c-SRC | 171 | 342 |
| LYN | 200 | 400 |
| PDGFRα | 718 | 1436 |
| JAK1 | >10,000 | >10,000 |
| JAK2 | >10,000 | >10,000 |
| PI3K | >10,000 | >10,000 |
| PLK1 | >10,000 | >10,000 |
| SYK | >10,000 | >10,000 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree