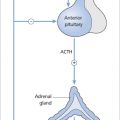
Acquired hypothyroidism may be primary or central as mentioned above. Primary hypothyroidism may be due to chronic autoimmune (Hashimoto’s) thyroiditis, iatrogenic causes (e.g. drugs, thyroidectomy, radioiodine), iodine deficiency/excess or thyroiditis.
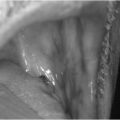
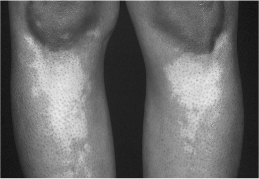
Chronic autoimmune (Hashimoto’s) thyroiditis is caused by cellular and antibody-mediated injury to the thyroid tissue. There are two forms, goitrous and atrophic, which have similar pathophysiology but are different in the extent of thyroid follicular cell hyperplasia, lymphocytic infiltration and fibrosis. Chronic autoimmune thyroiditis is usually but not always permanent. These patients are more likely to have a personal or family history of other autoimmune conditions, such as Addison’s disease and type 1 diabetes mellitus, vitiligo (Fig. 2.1), pernicious anaemia and premature ovarian failure. An increased incidence of Hashimoto’s thyroiditis is also found in those with Down’s or Turner’s syndrome.
Drugs such as the antithyroid drugs carbimazole and propylthiouracil (used to treat hyperthyroidism) may cause hypothyroidism. Amiodarone is an iodine-containing drug and may cause both hypothyroidism (see below) and thyrotoxicosis (see Chapter 3). Other drugs that may cause hypothyroidism include lithium, alpha-interferon and interleukin-2. Patients on these drugs should have their serum TSH checked every 6–12 months.
T4 has a half-life of 7 days, and hypothyroidism occurs about 2–4 weeks following total thyroidectomy. After subtotal thyroidectomy for the treatment of Graves’ disease, hypothyroidism occurs within the first year in the majority of patients. The annual risk of hypothyroidism in those who are euthyroid at 1 year is 0.5–1%. Some patients become transiently hypothyroid after 4–8 weeks but recover several weeks or months later.
Figure 2.1 Vitiligo in a patient with Hashimoto’s thyroiditis.
Following radioiodine therapy for the treatment of Graves’ disease, the majority of patients become hypothyroid within the first year. The rest have a 0.5–2% annual risk of hypothyroidism. Some patients with toxic multinodular goitre or thyroid adenomas who receive radioiodine therapy also become hypothyroid.
External irradiation of the neck may result in hypothyroidism with a gradual onset. Many patients develop overt hypothyroidism after several years of subclinical hypothyroidism.
Iodine deficiency and excess can both cause hypothyroidism. Iodine deficiency is the most common worldwide cause of hypothyroidism and is more prevalent in mountainous areas. Iodine excess can also result in hypothyroidism by inhibiting iodine organification and T4 and T3 synthesis (Wolff–Chaikoff effect).
In postpartum and subacute thyroiditis, transient hypothyroidism (lasting weeks to a few months) may follow transient thyrotoxicosis.
Rarer causes include infiltrative diseases, for example fibrous (Reidel’s) thyroiditis, haemochromatosis, sarcoidosis, amyloidosis, leukaemia, and consumptive hypothyroidism due to an ectopic production of the type 3 deiodinase in vascular and fibrotic tumours, which metabolizes T4 to reverse T3.
Clinical presentations
Hypothyroidism often has an insidious and non-specific onset. Patients may present with fatigue, lethargy and cold intolerance. Clinical presentations of thyroid hormone deficiency result from a generalized slowing of metabolic processes and an accumulation of hydrophilic glycosaminoglycans in the interstitial spaces of the tissues (myxoedema). Box 2.2 summarizes the clinical presentations of hypothyroidism in adults and possible explanations for these manifestations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree