Chapter 26 Hypersensitivity (Type IV)
• DTH reflects the presence of antigen-specific T cell-mediated inflammation.
• There are three variants of type IV hypersensitivity reaction – contact, tuberculin, and granulomatous.
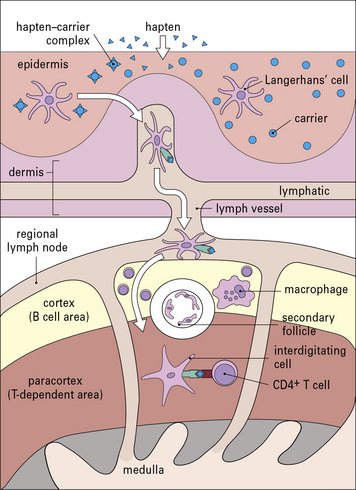
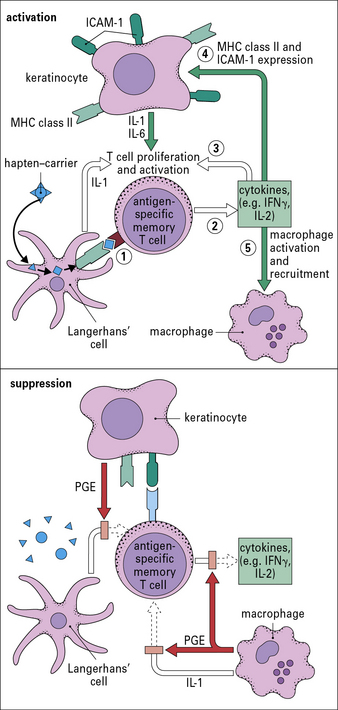
• Contact hypersensitivity occurs at the site of contact with an allergen. Sensitization occurs when skin dendritic cells internalize and process epicutaneously applied hapten and migrate to the draining lymph nodes where they activate antigen-specific T cells. On re-exposure to antigen, cytokines produced by skin cells (e.g. keratinocytes, Langerhans’ cells), recruit antigen-specific, and also non-specific T cells, and macrophages.
• Tuberculin-type hypersensitivity is induced by CD4 T cell responses to soluble antigens from a variety of organisms. It is useful as a diagnostic test to detect infection with a number of infectious agents.
• Granulomatous hypersensitivity is clinically the most important form of type IV hypersensitivity. Persistence of antigen leads to chronic T cell activation, differentiation of macrophages into epithelioid cells, and their fusion to form giant cells. This granulomatous reaction results in tissue pathology. Granuloma formation is driven by T cell activation of macrophages, and is dependent on TNF. Inhibition of TNF leads to breakdown in granulomas.
• Many chronic diseases manifest type IV granulomatous hypersensitivity. These include tuberculosis, leprosy, schistosomiasis, sarcoidosis, and Crohn’s disease.
Delayed hypersensitivity
For example, the late-phase IgE-mediated reaction may peak 12–24 hours after contact with allergen, and TH2 cells and eosinophils contribute to the inflammation as well as IgE (see Chapter 23).
There are three variants of type IV hypersensitivity reaction
Three variants of type IV hypersensitivity reaction are recognized (Fig. 26.1):
• contact hypersensitivity and tuberculin-type hypersensitivity both occur within 72 hours of re-exposure to antigen;
• granulomatous hypersensitivity reactions develop over a period of 21–28 days – the granulomas are formed by the aggregation of macrophages and lymphocytes and may persist for weeks – this is the most important type of type IV hypersensitivity response for producing clinical consequences.
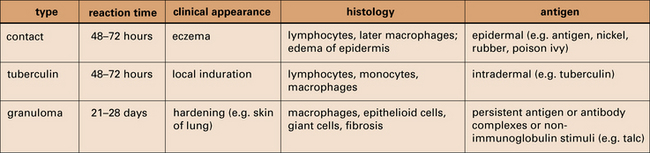
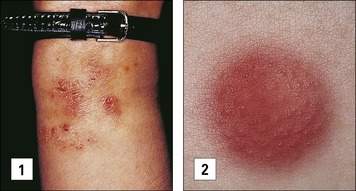
Fig. 26.1 Delayed hypersensitivity reactions
The characteristics of type IV reactions comparing contact, tuberculin, and granulomatous reactions.
Contact hypersensitivity
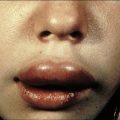
Contact hypersensitivity is characterized by an eczematous skin reaction at the site of contact with an allergen (Fig. 26.2). Sensitizing agents for humans include metal ions, such as nickel and chromium, many industrial chemicals including those in rubber and leather and natural products present in dyes, drugs, fragrances and plants, such as pentadecacatechol, the sensitizing chemical in poison ivy. This is distinct from the non-immune-mediated inflammatory response to irritants.
Sensitizing agents behave as haptens. Haptens are:
• low molecular weight chemicals (< 1 kDa) that are not immunogenic by themselves
• lipophilic and penetrate the epidermis and dermis where they bind covalently to cysteine or lysine residues in self proteins to form new antigenic determinants.
• metal ions, which chelate with self-peptides in the groove of MHC class II.
A contact hypersensitivity reaction has two stages – sensitization and elicitation
Dendritic cells and keratinocytes have key roles in the sensitization phase
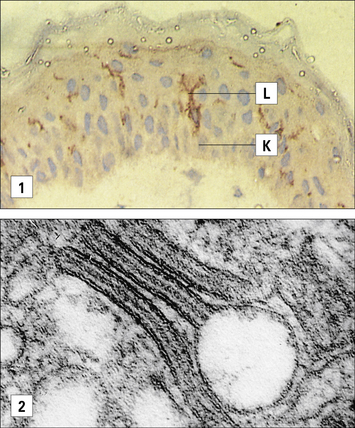
Antigen presenting cells (APC) in the skin include Langerhans’ cell (LCs), located in the suprabasal epidermis, and dermal dendritic cells (dDCs). Contact hypersensitivity is primarily an epidermal reaction, and epidermal LCs were considered to be the APC responsible for initiating contact sensitivity (Fig. 26.3). More recent studies have established that dDCs are essential for stimulating hapten-specific T cells.
Langerhans’ cells (see Chapter 2) are specialized DCs which extend dendritic processes throughout the epidermis, allowing them to sample environmental antigens. LCs express MHC class II, CD1 and the C-type lectin, langerin (CD207), which is responsible for the development of Birbeck granules, the cell membrane-derived organelle characteristic of LCs (see Fig. 26.3). The majority of dermal DCs are Langerin−, but there is a small population of Langerin+ dDCs, which are distinct from LCs, but also migrate rapidly to draining lymph nodes on exposure to sensitizers and activate hapten-specific CD8+ T cells. Both LCs and dDCs take up hapten-modified proteins by micropinocytosis but they also absorb lipid-soluble haptens, which modify cytoplasmic proteins. Under the influence of IL-1 and TNF secreted by keratinocytes and other cells, these DCs undergo maturation and increase expression of MHC and co-stimulatory molecules. Both LCs and dDCs are inactivated by ultraviolet B, which can therefore prevent or alleviate the effects of contact hypersensitivity.
Keratinocytes produce cytokines important to the contact hypersensitivity response
Activated keratinocytes produce a wide range of cytokines, including:
• TNF, IL-1, and granulocyte–macrophage colony stimulating factor (GM–CSF), which activate LCs and dDCs;
• IL-3 which activates LCs and co-stimulates T cell proliferative responses, recruits mast cells, and induces secretion of immunosuppressive cytokines, such as IL-10 and transforming growth factor-β (TGFβ). These dampen the immune response and may induce clonal anergy or immunological unresponsiveness in TH1 cells.
Sensitization stimulates a population of memory T cells
MHC class I-restricted CD8+ T cells are important in contact hypersensitivity responses in humans and mice and are the major effector cells for many allergens. For example, lipid-soluble urushiol from poison ivy enters the cytoplasm of APCs and haptened cytoplasmic proteins are processed through the MHC class I pathway, leading to the activation of allergen-specific CD8+ T cells. Hapten-specific CD4 T cells are also activated hapten–peptide conjugates in association with MHC class II molecules and become effector/memory CD4+ T cells, which contribute to the skin inflammation, or regulatory CD4+ T cells (Fig. 26.4).
Q. What effect will loss of CCR7 and CD62L have on T cell function?
A. CD62L promotes adhesion of lymphocytes to high endothelial venules and CCR7 allows the cells to respond to CCL21 expressed in secondary lymphoid tissues (see Fig. 6.15). Hence cells lacking these receptors will lose their propensity to traffic into lymph tissues.
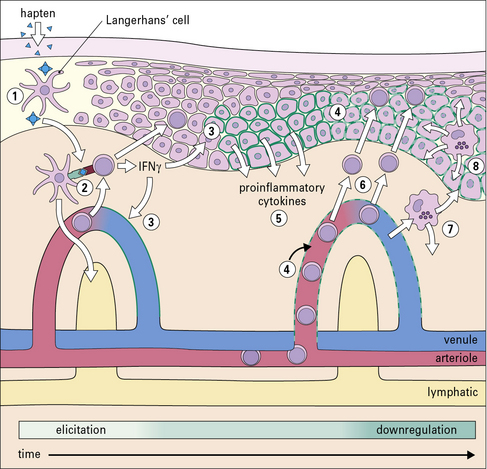
Elicitation involves recruitment of CD4+ and CD8+ lymphocytes and monocytes
The application of a contact allergen leads to:
• rapid expression of proinflammatory cytokines; and
• recruitment of effector T cells and monocytes to the site (Fig. 26.5).
TNF and IL-1 are potent inducers of endothelial cell adhesion molecules, including:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree