Introduction
Calcium, the most abundant mineral in the human body, has an essential role in many cellular processes such as enzymatic reactions and neuromuscular functions. It is also one of the building blocks of bone. Most of the body’s calcium, in fact, is in the skeleton, but the 0.3% that is found in the circulation is a critically important regulator of the function of other organs such as the nervous system and the heart. Calcium in the circulation is partitioned into three compartments: 40% is bound to proteins, mainly albumin; about 10% is complexed to ions such as citrate; and 50% is free or ionized, which reflects the physiologically active moiety. The total serum calcium concentration accurately reflects the ionized fraction unless there is an abnormality in the serum albumin or in pH. When the serum albumin is reduced, a correction is made by increasing the measured calcium value by 0.8 mg/dL for every 1 g/dL reduction in the serum albumin. Symptoms of hypercalcemia reflect, in part, the corrected serum calcium concentration. Hypercalcemia is defined by serum calcium levels above the normal range (e.g., 8.4 to 10.2 mg/dL). Most clinicians classify hypercalcemia into three grades: mild (less than 12 mg/dL), moderate (12 to 14 mg/dL), or severe greater than 14 mg/dL). The estimated prevalence of hypercalcemia in the general population is 1% to 3%. ,
Etiology/Pathophysiology
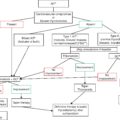
Parathyroid hormone (PTH) and vitamin D play important roles in calcium homeostasis. Secretion of PTH is regulated by the ionized calcium concentration. An increase in the ionized calcium concentration will abruptly reduce the synthesis and secretion of PTH. The vast majority of patients who present with hypercalcemia, about 90%, will have primary hyperparathyroidism (PHPT) or hypercalcemia of malignancy (HOM). In these two situations, the parathyroid system either recognizes (HOM) or does not recognize (PHPT) that the serum calcium is elevated, and PTH is either responsible for the hypercalcemia (PHPT) or is not responsible for the hypercalcemia (HOM). We refer to these two entities as PTH-dependent (PHPT) or PTH-independent (HOM) hypercalcemia. The remaining 10% of hypercalcemic individuals comprise a long list of other etiologies. As is the case for the two most common causes of hypercalcemia, the remaining etiologies are either dependent or independent of PTH.
PTH-DEPENDENT HYPERCALCEMIA
Besides PHPT, hypercalcemia due to excessive PTH secretion can be seen in the following conditions: chronic use of thiazide diuretics or lithium, end-stage renal disease (tertiary hyperparathyroidism), parathyroid cancer, or, very rarely, a tumor that secretes authentic PTH.
PHPT accounts for more than 90% of the cases of hypercalcemia in ambulatory settings. In the United States, PHPT has an estimated prevalence of 23 cases per 10,000 in women and 8.5 per 10,000 in men. PTH excess can arise from a benign, neoplastic change of one or more of the four parathyroid glands. Single parathyroid adenomas are the most common etiology of PHPT, accounting for approximately 80% of cases. Other causes include multiple adenomas (∼10%), hyperplasia of all four glands (less than 10%), and parathyroid carcinoma (less than 1%). Four-gland parathyroid disease is due to parathyroid cell hyperplasia. It can be sporadic or part of inherited syndromes, such as multiple endocrine neoplasia type 1 (MEN1), MEN2A, familial isolated hyperparathyroidism, and hereditary-jaw tumor syndrome. ,
Tertiary hyperparathyroidism is characterized by elevated serum calcium and PTH in patients with end-stage, chronic kidney disease. Decreased phosphorus excretion, failure of the kidneys to synthesize 1,25-dihydroxyvitamin D, and hypocalcaemia in patients with kidney disease are together responsible for secondary hyperparathyroidism. At this stage, particularly when the creatinine clearance falls below 40 mL/minute, the PTH level will be elevated but the serum calcium will be normal. The chronic and progressive effects of these biochemical imbalances may lead to a semi-autonomous condition, akin to PHPT, with hyperplasia of all four parathyroid glands and eventual emergence of frank hypercalcemia. When the serum calcium rises in this setting, the terminology changes from secondary (high PTH but no hypercalcemia) to tertiary hyperparathyroidism (high PTH and hypercalcemia). The estimated prevalence of secondary or tertiary hyperparathyroidism in patients with a glomerular filtration rate below 20 mL/minute per 1.73 m 2 is 80%. Tertiary hyperparathyroidism is reported in up to 30% of patients who are candidates for and have received kidney transplants.
Familial hypocalciuric hypercalcemia (FHH) is a clinical syndrome characterized by exceedingly low urinary calcium excretion in the setting of elevated serum calcium with inappropriately normal or elevated PTH. The genetic etiology in FHH, a mutation in the calcium-sensing receptor (CaSR) with reduced sensitivity to extracellular calcium, leads to a higher “set point” for calcium. Poor sensing by the mutant CaSRs in the kidney leads to excessive renal conservation and markedly reduced urinary calcium excretion. Three variants with generally similar phenotypes are known as FHH1, FHH2, and FHH3. FHH1 follows an autosomal dominant pattern of inheritance with complete penetrance, typically by the age of 30. Over 200 inactivating mutations in the CaSR have been described in FHH1. FHH2 is due to inactivating mutations in the GNA11 gene encoding the G-protein subunit α11. FHH3 is due to inactivating mutations in the AP2S1 gene encoding the adaptor-related protein complex 2, sigma 1 subunit (AP2σ). It is unusual for patients with FHH to develop end organ damage. The differentiation between PHPT and FHH usually rests with the familial inheritance pattern, the surfacing of the disease in young adulthood or before, and a urinary clearance ratio of Ca/Cr that is below 0.01. A cautionary note here is that many patients with PHPT, particularly those with low intake of calcium, can have urinary Ca to creatinine clearance ratios below 0.01. The diagnosis is unequivocally established by genetic analysis. It is important to recognize this genetic disease because patients with FHH should not undergo parathyroid surgery. It is not curative.
Lithium-induced hypercalcemia is characterized by elevated serum calcium levels with inappropriately normal or elevated PTH in patients. The mechanism remains elusive, but it has been suggested that lithium may alter the set point of CaSR. Thiazide-related hypercalcemia can also mimic PHPT. In both situations, stopping the drug, if possible, may lead to normalization of these biochemical indices. More often, however, the hypercalcemia and elevated PTH levels remain after stopping therapy for 3 to 6 months. The diagnosis of PHPT is then established. Some experts believe now that lithium and thiazides unmask latent PHPT that was not evident clinically prior to the administration of the drug. The majority of patients who are discovered to have hypercalcemia in the setting of lithium or thiazide use will be shown to have PHPT.
PTH-INDEPENDENT HYPERCALCEMIA
Suppressed PTH levels in the setting of hypercalcemia are consistent with non–PTH-mediated hypercalcemia as seen in patients with HOM, granulomatous diseases, milk alkali syndrome, hypervitaminosis D, and immobility.
HOM is a common complication of advanced cancer, with an estimated incidence of 20% to 30%. PTH-independent HOM is mediated through various mechanisms, including the development of focal osteolytic lesions; the secretion of parathyroid hormone-related peptide (PTHrP), also known as humoral HOM; or overproduction of 1,25-dihydroxyvitamin D (calcitriol). PTHrP-mediated HOM is usually associated with limited survival.
Overproduction of calcitriol is a well-recognized complication of lymphomas and granulomatous diseases and is thought to be driven by increased activity of 1-alpha-hydroxylase in macrophages. , Hypercalcemia in the setting of elevated calcitriol levels in patients with solid tumors has been described recently and was shown to be associated with failure to normalize serum calcium after antiresorptive therapy. Hypercalcemia, independent of PTH, can also be due to vitamin D toxicity as was reported in patients who took excessive amounts of an over-the-counter product, Soladek.
Presentation, Evaluation, and Diagnosis
The clinical presentation of hypercalcemia varies according to the degree of hypercalcemia and ranges from patients who are virtually asymptomatic to those who have severe symptoms. Often, the rapidity of onset of hypercalcemia rather than the absolute value of serum calcium determines the severity of symptoms related to hypercalcemia. The most likely etiology for asymptomatic, chronic hypercalcemia in the ambulatory setting is PHPT. The clinical manifestations of symptomatic hypercalcemia include polyuria, polydipsia, dehydration, neuropsychiatric disturbances, changes in sensorium, shortening of the QT interval, gastrointestinal symptoms, osteoporosis, kidney stones, and/or renal insufficiency. Although PHPT normally causes chronic mild (less than 12 mg/dL) hypercalcemia, some patients may be affected with severe symptoms. At times, these patients, against a backdrop of mild hypercalcemia, can become markedly hypercalemic. “Parathyroid crisis” is the term applied to these individuals. It is important to note that patients with PHPT can present with life-threatening hypercalcemia. This point is often not recalled in the setting of acute, symptomatic hypercalcemia in view of the fact that most patients with PHPT have only mildly elevated serum calcium levels. However, in a patient with an antecedent history of mild hypercalcemia, the emergence of acute, symptomatic hypercalcemia is most likely to be due to PHPT.
The first step after confirming the presence of hypercalcemia is to measure serum PTH levels. If serum PTH is elevated or inappropriately normal, it suggests PTH-mediated hypercalcemia. In patients without advanced kidney disease, and not taking thiazides or lithium, the differential diagnosis is rapidly narrowed to PHPT, FHH, or, rarely, a tumor producing authentic PTH. The latter possibility is so rare that it is practically not a consideration. A 24-hour urine measurement of the clearance ratio between calcium and creatinine usually settles any uncertainty between PHPT and FHH. This differential becomes important in younger individuals, particularly if there is a family history. Two points are worth repeating in this regard. FHH is a rare disease and there is almost 100% penetrance before the age of 30. In the typical postmenopausal woman who presents with mild hypercalcemia and an elevated PTH, it is all but certain that the correct diagnosis is PHPT. Measurements of vitamin D metabolites in PHPT will typically show levels of 25-hydroxyvitamin D that are in the low normal range. The 1,25-dihydroxyvitamin level will be in the high normal range or frankly elevated. It is usually not necessary to measure 1,25-dihydroxyvitamin D in the evaluation of PHPT. The serum phosphorus is typically in the low normal range, and in contrast to an earlier time, it is not often frankly low. , The evaluation in PHPT proceeds with bone mineral density, 24-hour urinary calcium and stone risk profile, and renal imaging to rule out nephrocalcinosis or nephrolithiasis. Guidelines for parathyroidectomy include any one of the following: serum calcium greater than 12.0 mg/dL; bone density T-score at lumbar spine, hip, or distal 1/3 radius less than or equal to 2.5; renal involvement (stones, renal calcifications, creatinine clearance less than 60 cc/minute); or age younger than 50. In patients who meet any one of these criteria and are planning to undergo parathyroidectomy, parathyroid localization studies are needed to identify abnormal parathyroid tissue. Localizing radiographic studies include sestamibi scintigraphy, sestamibi scintigraphy combined with single photoemission tomography (SPECT), SPECT combined with computed tomography (SPECT-CT fusion), and four-dimensional computed tomography (4D-CT).
HOM is the most common cause of PTH-independent hypercalcemia. Although HOM occurs in patients with cancer, it is uncommon to find patients with symptomatic hypercalcemia in whom the cancer is not known. After confirming PTH-independent hypercalcemia (i.e., the PTH level is undetectable), the next step is to determine the etiology of HOM by measuring PTHrP and 1,25-dihyroxyvitamin D in addition to a radiographic evaluation for osteolytic bone lesions.
Milk alkali syndrome is another cause of PTH-independent hypercalcemia that is due to ingestion of large amounts of calcium carbonate. Obtaining a thorough medical history of medications and supplements (including antacids) should be obtained during the evaluation of hypercalcemia.
PATHOPHYSIOLOGY OF SYMPTOMATIC HYPERCALCEMIA
The sequence of pathophysiologic events that lead to symptomatic hypercalcemia is common to virtually all causes of hypercalcemia. The central mechanism is the activated osteoclast, the bone-resorbing cell. It is activated in PHPT, in HOM, and even in vitamin D toxicity. The activated osteoclast excessively resorbs bone and releases calcium into the circulation. In patients with normal renal function who are not dehydrated, the calcium challenge will initially be met by increasing urinary calcium excretion. The hypercalcemia also impairs the kidneys’ water-conserving mechanisms, leading to polyuria. In turn, polydipsia follows. As the serum calcium rises further, the polydipsia cannot keep up with the polyuria, and anorexia develops. Dehydration follows and is worsened by vomiting, at times. Rapidly worsening hypercalcemia follows as the kidneys can no longer keep up with the need to excrete the additional calcium presented to them. Central nervous system features become prominent with lethargy and other indices of altered mental status.
MANAGEMENT
The degree of hypercalcemia along with associated symptoms determines the urgency of therapy. Symptoms are determined both by the level of the corrected serum calcium per se as well as its rate of rise. For the same hypercalcemic value, if the serum calcium has risen slowly, symptoms tend to be less severe.
Patients with mild hypercalcemia, such as in the typical patient with PHPT, tend to be asymptomatic. If symptoms are present, they tend to be nonspecific and do not require immediate treatment. In contrast, most patients with moderate to severe hypercalcemia present urgently and require immediate attention. The first step in the management of symptomatic hypercalcemia is to address the dehydration that inevitably accompanies symptomatic hypercalcemia. Intravenous fluid with isotonic saline is the fluid of choice because it helps to facilitate urinary calcium excretion. Within the first few hours 500 mL can be administered, followed by a more moderate rate of 150 to 200 mL/hour. Once hypovolemia is corrected, administration of furosemide can be considered in certain situations to further increase urinary calcium excretion. Furosemide is often not necessary in someone whose renal and cardiac functions are normal, but if there are concerns about the ability of the patient to handle the fluid load, the loop diuretic can be helpful. Another useful initial step is subcutaneous or intramuscular calcitonin, given as 200 units every 8 to 12 hours. Calcitonin reduces bone resorption, the central pathophysiologic feature of most severe hypercalcemic states. Although calcitonin works rapidly, within 12 hours, it is not very potent and will reduce the serum calcium by no more than 1 to 2 mg/dL. In addition, the effects of calcitonin are short-lived. Patients with severe hypercalcemia typically require more potent and long-lasting agents.
As the major “culprit” inciting the severe hypercalcemic state is the osteoclast, the most specific management approach is to use an inhibitor of osteoclast-mediated bone resorption. The bisphosphonates are the classic drugs used in this setting. Because intravenous or parenteral therapy is required, the two choices are pamidronate (30, 60, or 90 mg) or zoledronic acid (4 mg). Both agents are effective and generally follow the same time course, with the serum calcium falling 24 to 48 hours after administration. In the study of Major et al., zoledronic acid had a greater effect both in terms of the reduction of the serum calcium and the duration of the effect. Both agents do carry a warning about their use in patients with renal insufficiency. However, in the setting of acute hypercalcemia, and without preexisting renal disease, the renal dysfunction is usually “pre-renal” due to dehydration. It is not, therefore, an absolute contraindication to the use of bisphosphonates. However, renal function should be taken into consideration in the decision to use a bisphosphonate.
Another mechanism by which hypercalcemia is induced is by RANK ligand, a powerful bone-resorbing cytokine. It is often stimulated in the context of acute hypercalcemia. The RANK ligand inhibitor, denosumab, is, therefore, another option. , An advantage of denosumab (60 or 120 mg given subcutaneously; 120 mg is the approved dose for HOM) over the bisphosphonates is that renal dysfunction is not a contraindication. Denosumab, like the bisphosphonates, requires 24 to 48 hours for an effect to be appreciated.
Because both the bisphosphonates and denosumab are not immediately acting, a standard of many practitioners faced with this situation is to use combination therapy with calcitonin and either a bisphosphonate or denosumab. In this way one takes advantage of the rapid but weak effects of calcitonin while waiting for the more delayed but more powerful anticalcemic effects of pamidronate, zoledronic acid, or denosumab to manifest themselves.
If hypercalcemia is associated with elevated 1,25 dihydroxyvitamin D, such as occurs in some lymphomatous malignancies or granulomatous diseases, corticosteroids or other inhibitors of 1-alpha-hydroxylase (e.g., ketoconazole) can be considered. Multiple myeloma and some breast cancers may be responsive to glucocorticoids. In extreme situations, hemodialysis can be used in patients with life-threatening hypercalcemia, refractory to the approaches listed above.
Summary
Hypercalcemia is a common medical problem, occurring in 1% to 3% of the population. It can be classified into mild (less than 12 mg/dL), moderate (12 to 14 mg/dL), or severe (greater than 14 mg/dL). The causes are conveniently divided into those in which the hypercalcemia is due to PTH excess and those in which the hypercalcemia is an independent process associated with suppressed PTH. More than 90% of hypercalemia is due to primary hyperparathyroidism or hypercalcemia of malignancy. They both can be associated with life-threatening hypercalcemia. The approaches to the management of severe hypercalcemia include intravenous hydration with saline, furosemide (if necessary), and calcitonin with an intravenous bisphosphonate or subcutaneous denosumab.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree