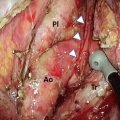
Fig. 15.1
Investigation algorithm for esophageal cancer at The University of Hong Kong
15.2.1 Staging
Clinical staging follows the American Joint Committee on Cancer Staging (AJCC) TNM classification system. In addition to endoscopy, bronchoscopy [3], percutaneous ultrasound of the neck with or without fine-needle aspiration (FNA) cytology, endoscopic ultrasonography (EUS) with or without fine-needle aspiration [4], and 2-(18F)-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (PET)/computed tomography (CT) scan are routinely employed.
Since the 1960s, bronchoscopic examination has been routine practice for patients with esophageal cancer at the authors’ institution, initially by rigid and later with flexible bronchoscope [5, 6]. This is especially important for tumors that are located in the middle and upper portions of the esophagus. In one study, the reported complication rate was 0.95 % (4 out of 525 patients). Airway involvement by tumor contraindicates surgical resection. In a handful of anecdotal cases, response to chemoradiation therapy resulted in disappearance of tumor involvement, leading to subsequent successful resection, but this is exception rather than the rule.
Percutaneous ultrasonography with or without fine-needle aspiration is crucial to delineate the nodal status of the cervical region, and this is routinely performed. Diagnosis of cervical nodal metastases is important from a therapy point of view. In the previous AJCC staging classification (sixth edition), cervical nodal metastases were regarded as stage IV disease and our policies have been to treat these patients with up-front chemoradiation followed by surgery if restaging demonstrates resectable disease.
We have been using endoscopic ultrasound (EUS) for staging since the 1990s [4]. The sensitivity and specificity of EUS in detecting the depth of esophageal involvement are 89 and 96 %, respectively, whereas the respective figures for nodal status are 85 and 86 % [4], results that are comparable with other reports [7–9]. In recent years we have been using the miniaturized ultrasound catheter probes (12.5 MHz mini probe), mainly because a substantial proportion of our patients have untraversable tumor stenosis for conventional dedicated radial endo-ultrasonic endoscope.
In Hong Kong, FDG-PET has gained popularity for esophageal cancer staging since about a decade ago; it is used in most of our patients although one limitation is that the scan is still not publicly funded so only contrast CT scans are used in the minority of patients who cannot afford a PET scan. In an early study, we found that the maximum standard uptake value (SUVmax) correlated with nodal status (N+ vs. N − disease) on PET scan, the T stage measured by EUS, the pathological T stage after surgical resection, the pathological overall stage, as well as the chance of an R0 resection [10].
In a more recent series of 244 patients from 2007 to 2012, we found that the SUVmax correlated with the T stage of disease; the mean SUVmax values for T1, T2, T3, and T4 tumors were 2.74, 4.55, 12.9, and 13.6, respectively. In addition, an SUVmax of 7.3 or more predicted a T3/T4 tumor with a sensitivity of 90.1 % and specificity of 95 %. For nodal metastases, our experience with PET scan has an accuracy of diagnosing positive nodal spread of 70.3 %. The tendency however is to underestimate false-negative nodes, while the predictive value of a metabolically active node is generally high [10]. For patients who undergo neoadjuvant therapy, our policy is to repeat a PET/CT at 4 weeks post therapy before a decision is made for surgical resection.
15.2.2 Patient Pretreatment Evaluation
Accurate tumor staging provides guidance for stage-directed therapy. In addition to tumor stage, careful pretreatment risk evaluation is important in selecting the appropriate patients for surgery.
The physiological reserve is an important factor to evaluate for potential surgical candidate. The assessment is generally based on surgeons’ experience and intuition rather than an exact science. Objective score systems are available to help the assessment of operative risk and patient selection [11, 12]. In our multivariate analysis of predictive factors for morbidity and mortality after esophagectomy, advanced age was predictive of both pulmonary complications and postoperative death. Patients with tumor location in the superior mediastinal segment were also at risk of pulmonary complications [13]. In addition to routine blood tests for work-up, specific tests would include a pulmonary spirometric function test and, in selected patients, an echocardiogram and coronary angiography or stress thallium test. In our experience, there is not much in general that can be done to improve the existing physiological fitness of patients, with perhaps the exception of cardio-revascularization by angioplasty or stenting in those with critical coronary artery stenosis. In such patients, double antiplatelet agents such as aspirin and clopidogrel may be required after coronary intervention. Neoadjuvant therapy is often chosen in these patients with the purposes of: first with an aim to downstage tumor, second it would allow the patient to recover from the cardiac procedure, and third, antiplatelet agents, especially clopidogrel, can then be stopped during the time of esophagectomy.
15.3 Treatment
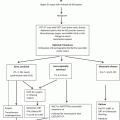
Surgical resection and radical radiotherapy used to be the only two treatment options for esophageal cancer. Advancement in endoscopic technology has made endoscopic treatment for early cancer possible. Improvement in chemotherapy and radiotherapy also increases the choice of therapeutic options. The management algorithm for ESCC at The University of Hong Kong is shown in Fig. 15.2.
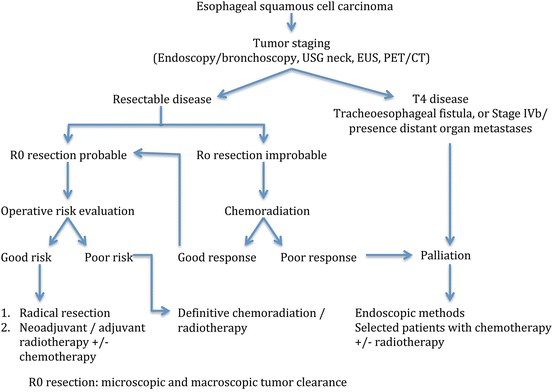

Fig. 15.2
Management protocol for esophageal squamous cell carcinoma at The University of Hong Kong
15.3.1 Endoscopic Treatment for Early Cancer
Early ESCC is defined as tumor that is limited to the mucosa or submucosa. In Hong Kong, most ESCC patients are diagnosed at an advanced stage. The number of patients suitable for endoscopic treatment is therefore small. The indications for endoscopic treatment generally follow the guidelines from Japan [14]. Distinguishing m1 and m2 disease (where chance of nodal metastases is negligible) from deeper lesions is often difficult, and we practice endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) to assess the lesions in detail pathologically before deciding on further treatments.
15.3.2 Neoadjuvant or Adjuvant Treatment
Neoadjuvant and adjuvant therapy for esophageal cancer has been controversial. Policies on their use vary widely in different countries. Preoperative chemotherapy is the standard of care in Japan and is commonly used in the United Kingdom [15–17], while in the United States chemoradiation is more widely practiced [18–20]. With the recent published CROSS trial from Europe, neoadjuvant chemoradiation is another standard strategy in many centers [21].
In Hong Kong, we have investigated different treatment strategies in treating esophageal cancer in addition to surgical resection alone. An early randomized trial looked at the impact of postoperative radiotherapy after esophagectomy. It was found that while postoperative radiotherapy did not lead to overall improvement in survival, in those with palliative resections, the addition of radiotherapy reduced the chance of death from local-regional recurrence, especially from tracheobronchial recurrence [22]. The technique of radiotherapy was suboptimal by modern standard, the fractionation was high (3 Gy per fraction), and a few deaths resulted from the deleterious effects on the gastric conduit, even including perforation. This might have affected the overall survival results. Postoperative radiotherapy is not widely practiced worldwide, perhaps with the exception of some centers in China, where improved survival can be shown in selected patient populations [23, 24].
In the early 1990s, our focus shifted to preoperative chemotherapy. A randomized trial compared esophagectomy alone and two courses of preoperative cisplatin and 5-FU was carried out. Again overall survival benefit could not be demonstrated. A pathological complete response rate of 7 % was achieved, and in those who responded well to chemotherapy, survival was superior to those who had surgery alone. Unfortunately this was offset by those who responded poorly, whose survival was worse than the controls [25]. Attempts were made to identify predictors of response, but none was found to be reliable [26].
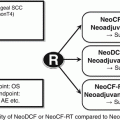
Disappointed by the results of chemotherapy, chemoradiotherapy as neoadjuvant therapy has been investigated since the mid-1990s. For most patients, the most common chemotherapy regime is cisplatin at 100 mg/m2 on day 1 and then day 22 and continuous infusion of 5-fluorouracil at 500 mg/m2 per day for 5 days from days 1 to 5 and days 22 to 26. Radiotherapy was given concurrently with a dose of 40 Gy at 2 Gy per fraction. With this approach, tumor downstaging of 75 % can be achieved. Pathological complete response rate in the primary tumor is 45 % and overall pathological complete response rate (including negative nodes) is 31 % [27]. In a study of 175 patients received neoadjuvant chemoradiation followed by surgery were studied; the 5-year survival rates of patients of complete pathological response versus those with residual tumor cells were 61.6 versus 30.4 %, p < 0.01 (Fig. 15.3). The survival of these patients based on gender and respective pathological stages was shown in Figs. 15.4 and 15.5, respectively. In more recent years, the technique of radiotherapy has improved; often intensity-modulated radiation therapy (IMRT) and 3-D conformal radiotherapy planning are used replacing the traditional AP opposing radiation field. The choice of chemotherapeutic agents has widened to include taxanes as well, in response to the positive results of the CROSS trial.

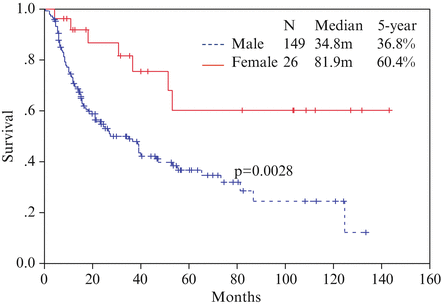
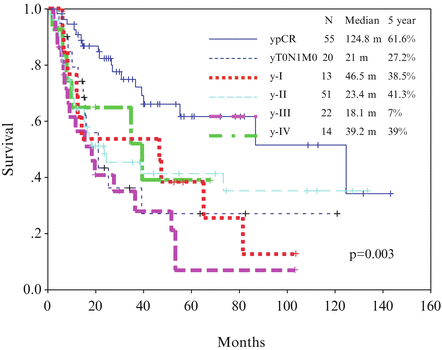

Fig. 15.3
Survival curve for patients with pathological complete response (ypCR) and other stages of disease

Fig. 15.4
Survival curve with gender as a predictor of survival

Fig. 15.5
Survival curve of ypTNM stage for patients treated with neoadjuvant chemoradiation followed by surgery
A historical cohort comparison on patients treated with surgery alone and surgery with neoadjuvant chemoradiation demonstrated that the latter had better overall survival [28]. The adoption of chemoradiation allowed better patient selection for curative surgery and resulted in more R0 resection by tumor downstaging [28].
The timing of surgery after chemoradiation is an important consideration; when the interval between chemoradiation and surgery is short, the resultant pathological response rate may be affected because the tumor has not had a chance to degenerate, tissue inflammation may still be severe, and patients often need some time to recover physically from the treatment. On the other hand, if the interval is left too long, fibrosis may ensue which may make dissection more difficult. More importantly, tumor may have more chance to regrow and metastasize. Our policy is to restage the patients at 4 weeks post therapy, including endoscopy and PET/CT scan. Surgery is then performed around 6–10 weeks post therapy.
In 107 ESCC patients who received neoadjuvant chemoradiation, the impact of the interval to surgery was studied. Patients were divided into two groups using 64 days from the end of therapy (median interval). When an R0 resection could be performed, the 3-year survival of the early surgery group was 71.7 % compared to 56.5 % of the delayed surgery group (p = 0.023). Postoperative morbidity and mortality rates were not affected by the timing of surgery [29]. Our previous data had also demonstrated the safety of neoadjuvant chemoradiation with regard to postoperative morbidity rates [13, 28]. The relationship between survival and interval to surgery after neoadjuvant therapy is an interesting observation, but the data require confirmation in a larger cohort. This factor needs consideration when surgery is planned.
The applicability of the AJCC staging system in the post neoadjuvant chemoradiation setting has also been questioned. In one study, we showed that in patients without neoadjuvant therapy, there was an orderly relationship between the chance of finding nodal metastases and advancing pT stage. This was no longer true after chemoradiation. Applying the same TNM staging system may not be accurate enough to provide prognostic information [30]. Instead the percent of residual viable cells in the primary tumor and nodal status were independent prognostic factors [27], while ypT stage was not. The cutoff point on the percent of residual viable cells and the interplay between other prognostic factors for better prognosis stratification warrant further investigation.
One major consideration of neoadjuvant therapy is how best to predict response. Chemotherapy and radiotherapy are not without morbidities, and subjecting patients to such treatments without significant effect will potentially cause harm, delay surgery, and increase the chance of tumor metastases while waiting for definitive treatment. No reliable clinical predictors exist. The use of PET scans to assess response during the early part of neoadjuvant therapy has some promise, but most reports to date studied chemotherapy-treated patients with substantial number being adenocarcinomas [31, 32]. At the authors’ institute, work is being carried out to develop a blood-based assay to predict response, for example, measuring serum microRNAs release from tumor cells. Further results are awaited.
15.3.3 Surgery
Most patients seen at the authors’ institute have advanced disease with comorbidities. Early cancers are uncommon and therefore most who come to surgery will have had neoadjuvant chemoradiation. These have to be taken into account when operative strategies are planned.
15.3.3.1 Cervical Esophageal Cancer
Cervical ESCC justifies separate consideration. It accounts for 2–10 % of all esophageal carcinomas, and by convention this cancer is treated by pharyngo-laryngo-esophagectomy (PLE) with or without adjuvant radiotherapy. PLE with one-stage gastric pull-up was first described by GB Ong in 1960 from The University of Hong Kong [33]. The original description of PLE involved a thoracotomy for esophageal mobilization. This was later modified so that a transhiatal approach was used without a thoracotomy. This was again changed when video-assisted thoracoscopic surgery (VATS) became available [34, 35].
In recent years, laryngeal preservation has been the aim of treatment and definitive chemoradiation therapy is increasingly used. In addition, the availability of the technique of free jejunal graft to replace the cervical esophagus has also reduced the need for PLE. The annual average number of PLE performed in the authors’ center dropped from 15 to around 6 [36]. Currently our practice is to offer patients a definitive chemoradiation for cervical ESCC. Surgery is performed in those who refuse nonoperative treatment and in those with contraindications for chemoradiation and for salvage of treatment failure or recurrent disease. Free jejunal graft for reconstruction is used for those with primary hypopharyngeal cancer or those with limited involvement of the cervical esophagus; otherwise, a PLE will be performed.
While the policy of up-front definitive chemoradiation is widely practiced, our data showed that this type of strategy was not without drawbacks. Chemoradiation-related complications included mucositis, bilateral vocal cord palsies, esophageal stricture, carotid blowout, hypothyroidism, and hypoparathyroidism. Persistent dysphagia affected 29 % of patients and 38 % eventually required surgery for salvage [36].
On the other hand, the outcomes of PLE have significantly improved over the last few decades [37–40]. Both the anastomotic leak and mortality rates were reduced to 9 % [37]. Improvements of surgical technique including thoracoscopic esophageal mobilization and better perioperative care and patient selection all contributed to better results [34, 35]. Free jejunal graft as an esophageal substitute has a failure rate of 2 % and anastomotic leak rate of 4.6 % [41, 42]. Optimal treatment strategy for cervical esophageal cancer patients needs individual consideration.
15.3.3.2 Intrathoracic Esophageal Cancer
The Approach to Resection
Our preferred surgical approach is a transthoracic one. This is related to the fact that most of our tumors are squamous in type, located proximally in the esophagus, and advanced in nature and often neoadjuvant therapy has been given. We performed a randomized trial comparing transthoracic and transhiatal approach for lower third tumors [43]. No significant difference was found; the trial sample size was too small to be able to demonstrate any difference. The study was terminated because our focus then shifted to minimally invasive esophagectomy.
In Hong Kong, the application of minimally invasive surgical technology in esophagectomy commenced in the mid-1990s [44]. At the initial stage, thoracoscopic esophageal dissection was applied in lieu of open thoracotomy or transhiatal mobilization of the esophagus in PLE [34]. Then it was applied to intrathoracic esophageal cancers as well. Thoracoscopic esophageal mobilization with lymphadenectomy was carried out combined with open laparotomy for gastric conduit preparation for cervical esophagogastrostomy [45]. Our early hypothesis was that minimally invasive method had its maximum benefits in those with high risk of surgery and such patients were preferentially selected. The early results though acceptable were not impressive [44]. This was because of the combination of high-risk patients, immature techniques, and suboptimal instrumentation. The uptake of minimally invasive esophagectomy was thus slow. It was not until 2006 that we changed our policy and applied the operation more unselectively. In addition, laparoscopic gastric mobilization was introduced so that the whole procedure became totally minimally invasive (MIE). Our techniques have also improved together with better instruments.
To date we have performed such procedures in over 220 patients. MIE is the approach in about 65 % of our patients; in over 40 % of these patients prior neoadjuvant therapy has been applied (and in recent years, over 60 % of patients have had neoadjuvant chemoradiation). VAT esophagectomy with laparotomy was performed in 108 patients, totally MIE in 112. The median thoracoscopy time was 135 min, blood loss 300 ml. Our conversion rate (VAT) was 18 %, reflecting our policy of unselected choice of patients. In no case was conversion urgent because of complications; most were related to extensive lung adhesions or advanced post chemoradiated tumors that were found to be unsafe to dissect. Pulmonary complications occurred in 17 % of patients, anastomotic leaks in 4.5 %, and hospital mortality rate in 1 %. Two patients died, one from radiation pneumonitis postoperatively and another from ischemic gastric conduit which required further surgery. Although this was successfully salvaged surgically, the patients succumbed from postoperative myocardial infarction [unpublished data]. These results compare well with our open surgery results. Oncologically the number of nodes harvested was not different from open esophagectomy; both methods retrieved approximately 40 nodes per patient.
A recently published European randomized controlled trial demonstrated the benefit of minimally invasive esophagectomy in lowering pulmonary complications. Postoperative quality of life was also superior [46]. It is anticipated that the uptake of MIE will become more widespread around the globe [47].
Extent of Resection and Lymphadenectomy
A curative (R0) resection implies histological clear proximal, distal, and lateral margins. There is propensity of ESCC to have intramural and submucosal spread. Increasing the length of resection margin reduces the chance of a histologically positive resection margin. We advocate an in situ proximal resection margin length of 10 cm to allow a less than 5 % chance of anastomotic recurrence [48]. In our study, 28 (5.3 %) out of 524 patients developed anastomotic recurrence; the length of the axial resection margin correlated with the chance of anastomotic recurrence [49]. A negative margin, however, may not totally preclude anastomotic recurrence. In our experience, a positive resection margin occurred in 7.5 % of patients compared to 4.9 % in those with negative margin [48].
On lymphadenectomy, although extended mediastinal lymphadenectomy is practiced, cervical nodal dissection is not routinely performed at our institution. In patients with overt cervical nodal metastases, our policy is in general to treat with up-front chemoradiation before surgical resection. In a study of 109 patients with cervical nodal metastases proven on ultrasound-guided FNA, survival was compared among those with stage IV disease by virtue of cervical nodal disease (AJCC sixth edition) and those with systemic metastases. The former group had significantly longer survival compared to the latter group; median survival was 9.8 versus 3 months. More importantly, in those with up-front chemoradiation and then esophagectomy, a median survival of 35 months was achieved [50]. In our experience, cervical lymphadenectomy after neoadjuvant chemoradiation often yields negative nodes on histological examination, but dissection around the recurrent laryngeal nerve may lead to a higher rate of vocal cord palsy. However, nodal dissection should still be performed because of the unreliable means of confirming absence of metastases after chemoradiation.
In patients with no evidence of cervical nodal metastases on preoperative work-up including PET/CT scan and ultrasound, routine cervical lymphadenectomy is not performed. Neck dissection can still be performed later should recurrence occur locally in the cervical region. In our study of recurrence pattern after esophagectomy without routine cervical lymphadenectomy, isolated recurrence in the neck was uncommon. We studied 108 patients who underwent curative resection for ESCC; 56 (52 %) of them developed recurrence. There were 12 patients who had cervical nodal recurrence; only 4 of whom had isolated recurrence in the neck. This implies that cervical nodal recurrence, if present, tends to be found together with other sites of recurrences, thus reducing the benefits of cervical lymphadenectomy [49].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree