Fig. 10.1
Evolution of Gleason grading system
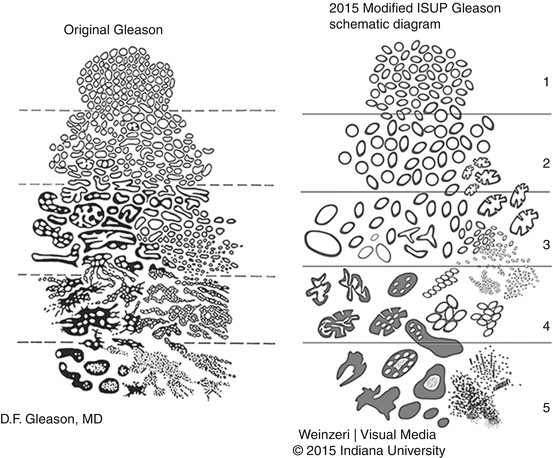
Fig. 10.2
Comparison of the original Gleason grading and the latest modifications (With permission, J Epstein et al. Am J Surg Pathol Volume 40, Number 2, February 2016)
Gleason grading system is based on the architectural patterns of the tumor, and taking in consideration of heterogeneity of prostate cancer, it includes the sum of the most common and the second most common patterns. The grading system encompasses grades from 1 to 5, from well circumscribed uniform glands (grade 1) to the poorly differentiated tumor composed of solid sheets and/or single cells or areas of central (comedo) necrosis (Fig. 10.1). Initial description of the system was based on a study of 270 patients from the Minneapolis Veterans Administration Hospital, and the study was expanded to 463 men in 1967 and 1032 men in 1974 [6–8]. These initial studies included men mostly with advanced disease with either extraprostatic extension or metastatic disease at the time of diagnosis. The strength of this grading system is that it is relatively simple and fairly easily applied with reasonable concordance (reproducibility). In addition, it is one of the most powerful prognostic predictors in Pca, predicting pathological findings in RP, biochemical failure, local and distant metastasis after therapy, and Pca-specific mortality, and is one of the key parameters used in therapy planning, especially for decisions regarding active surveillance versus definitive therapy.
The current guidelines of the Gleason grading system , including the most recent updates which were introduced in 2014 at a meeting attended by pathologists with expertise in prostate pathology and groups of oncologists and urologists, include elimination of GS 2 (1 + 1) regardless of the specimen type and limiting GS 2–4 diagnosis to rare cases in which the cancer is diagnosed in transurethral resection (TURP) specimens where the circumscription of the tumor nodules can be observed [6] (Fig. 10.2).
It also includes expansion and better definition of Gleason grade 4 category. In Gleason’s original descriptions, grade 4 group included only “large clear cells growing in a diffuse pattern resembling hypernephroma; may show gland formation” (Fig. 10.1). Later modifications included raggedly infiltrating fused glands and glands that are not single and separate but coalesce and branch. The 2005 consensus further expanded the category to include most cribriform glands (except “small cribriform glands with regular contour and round evenly distributed lumina” still regarded as Gleason grade 3) and ill-defined glands with poorly formed glandular lumens or “poorly formed” glands.
In 2014, all cribriform glands regardless of the shape and size and glands with glomeruloid architecture, considered a variant of cribriform architecture , were added to the Gleason grade 4 category (Figs. 10.2 and 10.4). When compared with all the different patterns that make up Gleason grade 4 group, cribriform growth pattern has been shown to predict adverse clinical outcome parameters such as postoperative metastasis and disease-specific death [12, 13].
Distinguishing tangentially sectioned Gleason grade 3 glands that appear poorly formed from the truly poorly formed glands has been a great challenge for pathologists (Figs. 10.3 and 10.4). Although it was noted that “more than occasional poorly formed glands” must be seen for a tumor to be classified as Gleason grade 4 in the latest consensus meeting to avoid overcalling of tangentially cut glands, attempts have been made to determine the morphologic and quantitative criteria to overcome the ambiguity of “poorly formed” definition. Zhou et al. [14] studied interobserver reproducibility of diagnosing “poorly formed glands” on core needle biopsies among urologic pathologists and came up with recommendations to consider only cancer glands with no or rare luminal formation as “poorly formed” and to have at least ten “poorly formed glands” that are not immediately adjacent to well-formed glands to be considered Gleason pattern 4. This issue is of utmost important since presence of Gleason grade 4 pattern is among the criteria used to make decisions, namely, if a patient is going to be included in active surveillance programs or offered a definitive therapy, such as prostatectomy or radiation therapy. In a recent multi-institutional study involving an active surveillance cohort [15], the reproducibility of assigning a Gleason pattern 3 vs 4 or higher was worst for small foci with glands where the interpretation varied between tangentially sectioned Gleason pattern 3 vs poorly formed glands of Gleason pattern 4. The best interobserver agreement was in cribriform and glomeruloid Gleason 4 patterns [16] in a recent study among genitourinary pathologists. In line with the previous study by McKenney, hardly any consensus was reached on ill-formed and fused glands. More recent attempts to further optimize histologic grading of prostatic adenocarcinoma to decrease interobserver variance and provide consistent and more accurate prognostic stratification for the individual patient include attempts of incorporating individual architectural patterns into the grading scheme and assessing their relative contribution to the prognostic strength [17].
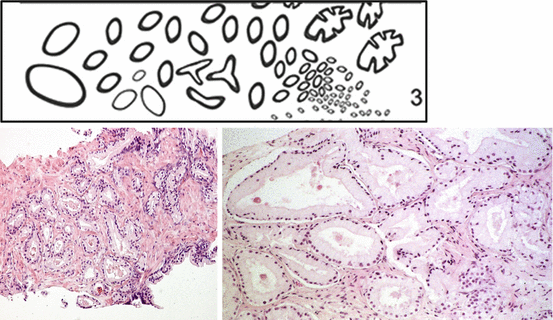
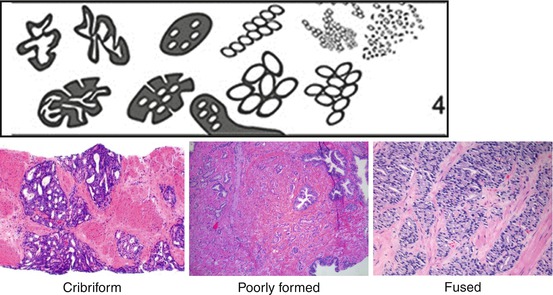

Fig. 10.3
Gleason grade 3 patterns

Fig. 10.4
Gleason grade 4 patterns
Gleason grade 5 patterns include single cells, solid tumor sheets, and cords of cells without any glandular differentiation as well as central (comedo) necrosis (Fig. 10.5). They generally do not present diagnostic challenges, unless the amount of single cells/cords of cells is limited.
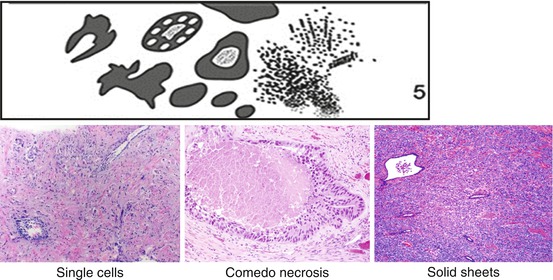

Fig. 10.5
Gleason grade 5 patterns
The most common practice is to give a Gleason score to each specimen of biopsy core(s) that is submitted in separate containers. For treatment algorithms, typically the highest Gleason score is used regardless of the volume of cancer in the particular core(s). In heterogeneous and multifocal tumors, this has the potential to overrepresent the high-grade component in the entire gland and place the patient in a higher-risk category. In a study by Aries-Stella et al., when using the highest biopsy Gleason score, the authors found that 60% of such patients had a downgraded Gleason score at prostatectomy [18]. As a result they concluded that a composite needle biopsy Gleason score combining relative proportions of each Gleason grade pattern in multiple contiguous positive biopsies correlated better with prostatectomy Gleason score in patients with heterogeneous needle biopsy Gleason scores (>2 different GSs or a two-step difference in GS between separate biopsies).
10.3 Grading Unusual Morphologies Variants of Pca
Overall the grading of histologic variants of Pca follows the conventional grading scheme, and except for the ductal carcinoma, most of these variants are assigned a Gleason score similar to the acinar carcinomas. Somewhat challenging patterns in this category include foamy gland and mucinous carcinomas and carcinomas with collagenous nodules and mucin extravasation. Previously, any carcinoma with extravasated mucin and mucinous (colloid) carcinomas (mucin extravasation occupying >25% of the tumor) were considered as Gleason grade 4. Recent studies have shown that mucinous carcinomas treated with RP are not aggressive and may even have better prognosis than the usual acinar carcinomas [19]. For these reasons, the latest consensus is that these carcinomas should be graded based on the pattern present after subtracting the mucinous component.
In contrast, all ductal carcinomas are graded as pattern 4 and pattern 5 if there is comedo necrosis. The ductal carcinoma component, even though it is mostly admixed with typical acinar carcinoma, is given a Gleason score of at least 8 (4 + 4) in keeping with the overall prognosis similar to other Gleason score 8 carcinomas.
Neither sarcomatoid nor small cell carcinomas should be graded due to their unique tumor biology and therapeutic implications, regardless of the amount of such components.
Intraductal carcinoma of the prostate (IDC-P), initially described by Kovi et al. in 1985 and McNeal in 1996, is described as dense cribriform glands with highly atypical epithelial cells that retain at least focally basal cells [20, 21]. Further studies, including by Guo and Epstein and Kimura et al., supported the adverse prognosis of IDC-P on biopsy and prostatectomy specimens and its association with high-grade invasive Pca [22–24]. Because of all the associations, some pathologists advocated grading of IDC-P; however, at the 2014 meeting it was the consensus not to grade it but to add a comment as to its invariable association with aggressive prostate cancer.
10.4 Tertiary Gleason Grade Pattern
The Gleason grading system is built on reporting the two most prevalent histological patterns as a sum score. More often than not, a tertiary or third most common pattern is encountered either in biopsies or prostatectomy specimens. Reporting this component especially if it is a higher-grade pattern may have different implications and purposes in needle biopsies and prostatectomies.
The current recommendation of including the higher tertiary grade component in the biopsies as the secondary grade is based on the assumption that the tumor has the biology of a higher-grade tumor, even though the amount of the higher-grade component is small in the biopsy, which may be due to a sampling error. The typical scenario of such a case is when the biopsy includes a tumor with a Gleason score 7, either 3 + 4 or 4 + 3 with a minor component of Gleason grade pattern 5. The final score in this case would end up being 8 (3 + 5) or 9 (4 + 5), respectively. This allows incorporation of the tertiary component in the risk stratification tools such as Partin tables and Kattan nomogram , which only allow primary and secondary patterns.
The presence of focal (less than 5% of the tumor volume) Gleason grade 5 has different implications in a prostatectomy since all tumor foci are available for review. It has been shown by Magi-Galuzzi et al. that in comparison the Gleason 7 (3 + 4) or 7 (4 + 3) with a tertiary pattern 5 was less likely to be organ confined and presented at a higher stage than the tumors with the same Gleason score but without the tertiary pattern 5 [25]. In these scenarios the Gleason score for the prostatectomy would be reported as 7 (4 + 3) with a tertiary 5, since the overall behavior of the tumor falls between Gleason 9 and 7.
10.5 Grade Groupings
With the modifications to the Gleason grading system , in recent years reporting of Gleason scores 2–5 has virtually vanished from general practice. This led to a shift in the grading system where originally the scores ranged from 2 to 10. Now the lowest score being 6 out of 10, giving the impression of the tumor to have intermediate prognosis, potentially causes patients unnecessary anxiety and overtreatment. There have been attempts to interpret the Gleason scores in groups of similar prognostic characteristics in the past; however, some of these groupings combined scores with wide variations in outcomes. For example, Gleason score 7 is considered as a homogenous group in many of these groupings, despite many studies showing a significantly worse prognosis for 4 + 3 than 3 + 4 [26–28]. In addition, combining scores 6 and 7, as some studies do, place tumors with almost uniformly excellent prognosis (3 + 3) and with a high likelihood of disease recurrence and progression (4 + 3) in the same prognostic category.
The newly accepted grade groupings that are included in the latest WHO 2016 edition of Pathology and Genetics : Tumours of the Urinary System and Male Genital Organs are proposed by Epstein based on data from Johns Hopkins Hospital [29]. It consists of five prognostically distinct grade groups, validated to accurately reflect prostate cancer biology. Grade group 1 in this new system includes cancers with Gleason score <6, with excellent prognosis, and now the grade designation of the tumor is better aligned with the lowest possible grade. Per consensus of the 2014 meeting, the new grades would, for the foreseeable future, be used in conjunction with the Gleason system [i.e., Gleason score 6 (3 + 3) (grade group 1)]. In a subsequent study of about 20,000 men from 5 different institutions who were treated by RP, grade groups strongly correlated with the risk of biological recurrence after surgery. In this study, large differences in recurrence rates between both Gleason 3 + 4 versus 4 + 3 and Gleason 8 versus 9 were found [30]. The proposed five-grade group system had the highest prognostic discrimination on both univariate and multivariate analysis. The major limitation was the use of PSA biochemical recurrence BCR as an end point as opposed to cancer-related death.
10.6 Prognostic Indicators
10.6.1 Volume
Prostate cancer is a heterogeneous disease with variable outcomes. One of the parameters suggested to be an important clinical predictor is the tumor volume. The assessment of tumor volume carries radically different implications in radical prostatectomy and needle biopsy. Whether tumor volume assessment in pathologically organ-confined (pT2) prostate cancer provides independent prognostic information beyond grade and stage is controversial. In early studies by Stamey and Humphrey, tumor volume correlated with metastasis, seminal vesicle invasion, extraprostatic extension (EPE), and grade [31, 32]. The latter study also showed that % of specimen involved by tumor independently predicted clinical recurrence. Since these studies have come out before PSA screening era, the mean tumor volume was fairly large 4.66 cc, and the mean Gleason score was 7.03 [31]. Nevertheless, in all the studies none of the tumor with volumes less than 0.5 cc progressed.
In the more contemporary studies, data on whether the tumor volume has independent prognostic significance in prostatectomy specimens are conflicting. One of the main reasons for this uncertainty is that there is not one agreed-upon method to estimate tumor volume. Among the presumably most precise methods used is the one that involves circling the tumor on glass slides and using computer-assisted planimetry or grid morphometric analysis to determine tumor volume. These methods are quite time consuming. Simpler methods such as visual estimation of tumor percentage and maximum tumor dimension (MTD) have the potential of wider use but due to their subjectivity and poor reproducibility fall short of providing uniform results. Other less frequently used methods include multiplying the largest tumor dimensions on the sections by the thickness of the tumor, using a grid to calculate tumor percentage, using number of blocks involved by tumor, calculating maximal area of the tumor, and determining the ratio of involved-to-uninvolved blocks. Another point of debate is whether to record the dominant tumor nodule(s) or total tumor volume. This point becomes more important in the tumors that would be in the clinically insignificant cancer category (less than 0.5 cc, no Gleason pattern 4, and organ confined) if only the largest of multiple small-volume tumors is reported as would be seen in a case with three separate tumor nodules (0.3, 0.2, and 0.1 cc) [33]. This variance in reporting total tumor volume may explain the variation in incidence of potentially insignificant cancers (ranging from 0.4–0.6% to 26–33%) in different institutions.
As a result of a consensus conference of the International Society of Urological Pathology , “some quantitative estimate of cancer volume should be undertaken” is recommended but “the nature of which being dependent on routine practice of with the pathology laboratory” [34].
Quantifying the amount of tumor in needle biopsies, on the other hand, provides one of the key data points used to make decisions on management options which increasingly in recent years includes active surveillance protocols. Given the importance of this parameter in management decisions, we should review the challenges associated with it. There are several ways to quantify cancer on biopsy. The simplest method is to estimate the percentage of the core(s) involved by carcinoma. While this assessment is easy, it is subjective and may be a cause for interobserver variability. Measuring cancer foci and total length of the biopsy cores may be more reproducible but also brings out other challenges such as how to measure two separate foci of carcinoma involving the same core but are separated by some amount of stroma. Among pathologists, there is no true consensus and there is variation in practice. Some pathologists measure each focus separately without including the intervening stroma, while others measure from one end to the other including the stroma. Multiple studies suggest that inclusion of the benign prostatic tissue in between the foci correlates more closely with the amount of tumor in the prostatectomy [35, 36].
There is limited number of studies comparing different quantitative methods in predicting pathologic stage or outcome. In one of the later studies, percentage of carcinoma in all cores was significantly and consistently stronger than other measures in all comparisons. When combined with preoperative PSA and Gleason grade on multivariate analysis, this measure improved prediction of pT3 and was independent of preoperative PSA and Gleason grade for risk of biochemical recurrence [37].
It is recommended that at a minimum the number of positive cores along with at least one other more detailed measurement such as the % of core involvement or length of cancer should be included in a biopsy report.
10.6.2 Extraprostatic Extension
Even more informative than the total tumor volume in a prostatectomy specimen is the extent of tumor, i.e., if the tumor extends into extraprostatic tissue (extraprostatic extension ) and/or into seminal vesicles. This information is reflected in the pathologic stage in the AJCC staging system as pT3a and pT3b, respectively. Extraprostatic extension is a well-established factor for adverse prognosis. However, definition and identification of extraprostatic extension can be challenging. Simply defined, extraprostatic extension is the tumor being present beyond the confines of the prostate gland [38]. This can be difficult to identify since the prostate gland lacks a discrete and microscopically well-defined prostate capsule. In addition, extraprostatic tissue present in different aspects of the prostate gland is different. For example, posterolateral soft tissue (superior neurovascular bundles ), as the name implies, includes abundant adipose tissue (unless a nerve-sparing prostatectomy) with blood vessels and nerves, whereas anteriorly, especially in the apex, the prostatic parenchyma blends in with bundles of striated muscle and only occasionally may include some amount of adipose tissue. The determination of what constitutes “beyond the confines of prostate” can be difficult when carcinoma involves the muscle anteriorly and bulges into the adipose tissue or extends along with the fibrous fingerlike projections into the neurovascular bundle. While finding cancer glands among striated muscle bundles anteriorly does not constitute extraprostatic extension, the latter is considered to be diagnostic of extraprostatic extension by many pathologists.
10.6.3 Seminal Vesicle Invasion
Seminal vesicle invasion is defined as involvement of the wall of seminal vesicles by carcinoma and in most instances is straightforward diagnosis. There are three ways of seminal vesicle invasion: 1, spreading along the ejaculatory ducts; 2, direct extension at the base or extraprostatic extension into the superior neurovascular bundle and then invasion into seminal vesicle wall; and 3, discontinuous involvement, which is quite rare.
A point of debate especially before the ISUP consensus publications was if the involvement of the intraprostatic portion of seminal vesicles also qualified as a pT3b disease [39]. In one of the few available studies on this subject, invasion of only the intraprostatic portion of the seminal vesicle or ejaculatory ducts was correlated with better survival rate than for those with extraprostatic seminal vesicle invasion [40]. Currently, only invasion of the extraprostatic portion of seminal vesicle is considered for staging.
10.6.4 Margins
Involvement of margins of resection of prostatectomy specimens is among the most common reasons for biochemical recurrence and is a significant predictor of BCR-free survival (93.8% in margin negative versus 79.9% in margin positive cases) and prostate cancer-specific mortality (PCSM) in a univariate analysis [41, 42]. After adjusting other clinicopathological variables in a multivariate analysis, it remained moderately associated with PCSM, while RP Gleason score and pathologic stage were strong predictors [43, 44]. The most common site of margin positivity is the apex where there is not much extraprostatic tissue and surgically removable tissue is limited due to the anatomical restrictions [45]. In the posterolateral aspect of the prostate, the amount of extraprostatic tissue in a prostatectomy depends on the type of surgical procedure: nerve spearing versus non-nerve spearing; thus, a positive margin can be either in the setting of extraprostatic extension or incomplete removal of the entire prostatic tissue. As may be expected, efforts to preserve the neurovascular bundles increase the probability of incomplete excision of the prostate resulting in more frequent positive margins. Positive margins at bladder neck, which is relatively uncommon, and anterior are also correlated with BCR, but several studies with opposite conclusions are have also been published [46, 47]. The type of surgical intervention, i.e., open radical prostatectomy (ORP) versus laparoscopic radical prostatectomy (LARP), may influence the incidence and location of positive margins: lower incidence in LARP and apical region in both LARP and ORP [48].
For pathologists a positive margin is defined as having carcinoma cells at the margins where the surgeon has cut across the tissue planes, which are marked with a special ink during gross examination. Since carcinoma cells being at the inked surface are the diagnostic hallmark, care needs to be given by the pathologist to recognize surface irregularities and disruptions that may not reflect true resection margins. Good communication with the surgeon may clarify many of these irregularities. In cases where carcinoma cells are not actually touching the ink but are only a few cells away may be classified as “carcinoma abutting” the margin. In a study by True et al., these cases behaved as positive margins; however, in other studies, close distance to the margins did not appear to correlate with disease recurrence or residual cancer [49–51].
On the other hand, the extent of tumor at the surgical margin has been shown to be correlated with disease recurrence in several studies [52, 53]. Determining and reporting the linear extent of positive margin in mm is the most common way pathologist reports the extent, while some may also classify the extent as “focal” versus “extensive.”
10.7 Treatment Effects on Histology
Prostate cancer treatments whether it be hormonal ablation or radiation therapy cause unique histologic changes in the carcinoma as well as the background nonneoplastic glandular tissue. Hormonal ablation causes, as expected, diffuse atrophy in the nonneoplastic glands with prominence of the basal cell layer (Fig. 10.6). Carcinomatous glands also shrink in size with vacuolization/clearing of the cytoplasm and nuclear pyknosis . Radiation therapy causes nuclear atypia and pleomorphism in addition to the diffuse atrophy in the nonneoplastic glands (Figs. 10.7 and 10.8). Stroma shows hyalinization and hypocellularity. Cancer glands become even more inconspicuous and appear mostly as single cells with vacuolated cytoplasm and eccentric nuclei or even empty spaces without any detectible nuclei.
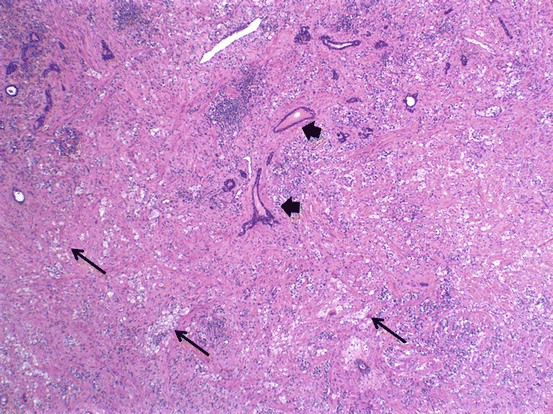
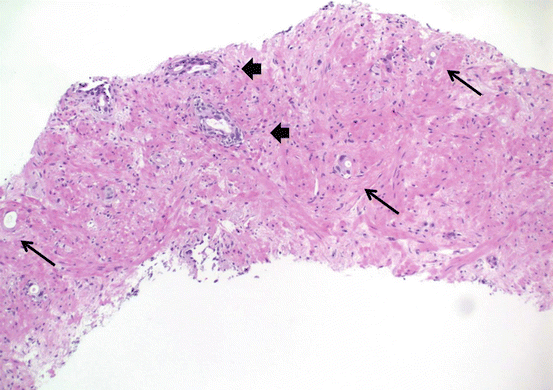
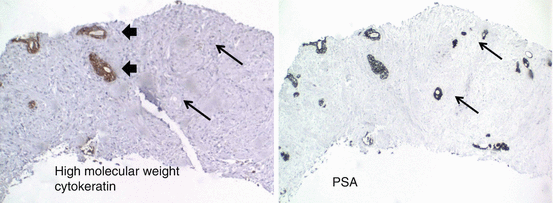

Fig. 10.6
Effects of hormonal ablation therapy on carcinoma and normal glands. Arrows = carcinoma glands; arrow heads = benign glands

Fig. 10.7
Effects of radiation therapy on carcinoma and normal glands. Arrows = carcinoma glands; arrow heads = benign glands

Fig. 10.8
Immunohistochemical profile of normal and carcinoma glands showing radiation therapy effect. Arrows = carcinoma glands; arrow heads = benign glands
Since all the described histologic changes in cancer are consistent with a high-grade morphology, Gleason grading no longer correlates with the disease severity and loses its prognostic prediction, and therefore by consensus no Gleason score is given to treated tumors. This creates a need for a new way of assessing the effect of therapy on the tumor and tumor burden, different than the volume estimation based on tumor area. One way is to assess the cellularity of the residual tumor. In a study by Murphy et al., the interobserver concordance was quite high for cellularity assessment in prostatectomies after treatment with abiraterone and enzalutamide, which may be a good candidate parameter to be measured if validation studies show that it correlates with the biology of the disease and the outcomes [54].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree