Optimal treatment of hilar cholangiocarcinoma depends on location of the cancer and extent of biliary and vascular involvement. Candidates for resection or transplantation must be evaluated and managed by a multidisciplinary team at a high-volume hepatobiliary center. Success requires absence of distant nodal or extrahepatic metastases and an adequate functional liver remnant with a negative ductal margin. Ipsilateral portal vein resection and reconstruction should be performed in patients with venous involvement. Neoadjuvant chemoradiation and liver transplantation is the best treatment option for patients with unresectable hilar cholangiocarcinoma without nodal or distant metastases and for patients with underlying chronic liver disease.
Key points
- •
Margin-negative cancer extirpation is the only curative strategy for hilar cholangiocarcinoma.
- •
Bismuth-Corlette type I and II cholangiocarcinomas are rare and are occasionally resectable without a concomitant liver resection; Bismuth-Corlette type III cholangiocarcinoma requires a concomitant hepatectomy for a margin-negative resection.
- •
Resection and reconstruction of ipsilateral portal vein for a margin-negative resection is feasible and safe. Portal vein resections without direct tumor involvement and arterial resections as a routine resectional component for hilar cholangiocarcinoma have been promoted in a few specialized centers, but survival benefit remains unclear.
- •
Bismuth-Corlette type IV cholangiocarcinoma and cancers with major vascular involvement should be considered for neoadjuvant chemoradiation and liver transplantation. Liver resection and liver transplantation are mutually exclusive treatment pathways without possibility of crossover.
- •
Operative treatment of hilar cholangiocarcinoma is associated with greater perioperative mortality than any other elective hepatobiliary operation.
Introduction
Hilar cholangiocarcinoma remains a major focus for hepatobiliary surgeons worldwide. Despite its relatively low incidence, aggressive tumor biology, lack of effective systemic therapy, and ongoing risks of hepatic failure and biliary sepsis have challenged the development of successful treatment strategies. Margin-negative resection of hilar cholangiocarcinoma remains the only potentially curative therapy.
The past few decades have witnessed the evolution of 2 dominant resection strategies: (1) concomitant biliary and hepatic resection and regional lymphadenectomy for patients in whom a negative margin is expected based on modern imaging and (2) liver transplantation after neoadjuvant chemoradiation for patients with unresectable hilar cholangiocarcinoma in the absence of identifiable metastases. In this article, we first discuss tumor pathology and staging, preoperative patient selection, and diagnostic techniques, and then focus on operative management and pertinent outcomes among patients with hilar cholangiocarcinoma.
Introduction
Hilar cholangiocarcinoma remains a major focus for hepatobiliary surgeons worldwide. Despite its relatively low incidence, aggressive tumor biology, lack of effective systemic therapy, and ongoing risks of hepatic failure and biliary sepsis have challenged the development of successful treatment strategies. Margin-negative resection of hilar cholangiocarcinoma remains the only potentially curative therapy.
The past few decades have witnessed the evolution of 2 dominant resection strategies: (1) concomitant biliary and hepatic resection and regional lymphadenectomy for patients in whom a negative margin is expected based on modern imaging and (2) liver transplantation after neoadjuvant chemoradiation for patients with unresectable hilar cholangiocarcinoma in the absence of identifiable metastases. In this article, we first discuss tumor pathology and staging, preoperative patient selection, and diagnostic techniques, and then focus on operative management and pertinent outcomes among patients with hilar cholangiocarcinoma.
Tumor pathology and staging
There are 3 pathologic subtypes of extrahepatic bile duct adenocarcinoma: sclerosing, nodular, and papillary. Sclerosing cholangiocarcinoma (>70%) is the most frequent subtype and is characterized by marked desmoplasia and neoplastic infiltration into surrounding tissues. Perineural and vascular invasion is frequent. Nodular cholangiocarcinoma (∼20%) shows local irregular infiltration into the bile duct. A combination of sclerosing and nodular features are observed with frequency and described as nodular-sclerosing. The sclerosing and nodular subtypes of cholangiocarcinoma have a predilection for radial and longitudinal extension along the mucosa and submucosa of the bile duct at times without extrabiliary evidence of tumor spread. Microscopic extension of sclerosing and nodular cholangiocarcinomas must be anticipated during resection. Papillary cholangiocarcinomas are rare (∼5%–10%) and are characterized by intraluminal mass with late transmural extension.
Preoperative evaluation and definition of the anatomic location and extent of hilar cholangiocarcinoma is critical for operative planning. To this extent, the Bismuth-Corlette classification ( Fig. 1 ) has been the most widely adopted system used to describe tumor location and biliary involvement. The strength and utility of Bismuth-Corlette classification is its ability to conceptualize hilar cholangiocarcinoma into an operatively approachable scheme. In practice, neoplasia is a dynamic process with a spectrum of extension that defies exact categorization.
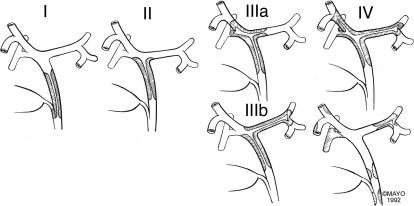
Patients with hilar cholangiocarcinoma at the level of the cystic duct, but below the bifurcation of the hepatic ducts (Bismuth-Corlette type I) or just at the bifurcation (Bismuth-Corlette type II) are best treated with resection of the extrahepatic bile duct, gallbladder, and regional lymph nodes and biliary reconstruction alone or with concomitant pancreaticoduodenectomy, depending on the distal extension of the cholangiocarcinoma. Occasionally, limited hepatic resection is required for type II cholangiocarcinoma. However, clinically, Bismuth-Corlette types I and II tumors are rare, and most patients with hilar cholangiocarcinoma have extension into the biliary bifurcation or the right or the left hepatic ducts, or both. Classification of hilar cholangiocarcinoma involving one or both hepatic ducts is Bismuth-Corlette IIIa for right segmental extension, IIIb for left segmental extension, and IV for bilateral segmental extension or diffuse multifocal disease. Resection is possible for IIIa and IIIb cholangiocarcinomas by right or left hepatectomy, respectively, if the vasculature to the expected hepatic remnant is either uninvolved or resectable and reconstructable.
Patients who have unresectable hilar cholangiocarcinoma are best treated by neoadjuvant radiotherapy with chemosensitization and liver transplantation. Although criteria for resectability vary widely, generally accepted criteria for unresectability are :
- 1.
Main portal or arterial involvement not amenable to reconstruction regardless of Bismuth-Corlette type
- 2.
Unilateral segmental biliary extension with contralateral vascular involvement not amenable to reconstruction (Bismuth-Corlette types IIIa and IIIb)
- 3.
Bilateral biliary involvement of secondary ducts (Bismuth-Corlette type IV)
- 4.
An inadequate future liver remnant whether caused by atrophy or insufficient response to growth stimulation (portal vein [PV] ligation or embolization)
In addition, underlying chronic liver disease (especially primary sclerosing cholangitis [PSC]) may preclude hepatic resection. PSC is an idiopathic cholestatic chronic liver disease associated with progressive inflammatory destruction and fibrosis of the entire biliary system and cirrhosis. PSC predisposes to cholangiocarcinoma throughout the biliary tract as a field defect with the potential for multiple cancers. Patients with PSC are best treated by neoadjuvant chemoradiation and liver transplantation rather than resection, even if they have otherwise potentially resectable tumors. Although the diagnoses of cholangiocarcinoma and PSC may be established at the same time, 7% to 15% of patients with PSC develop cholangiocarcinoma during their lifetime.
Adequate tumor staging has been historically challenging among patients with hilar cholangiocarcinoma. Unlike the previous editions, the seventh edition of the American Joint Committee on Cancer (AJCC) staging classification has separate staging schemes for intrahepatic, perihilar, and distal cholangiocarcinomas. The AJCC seventh edition T stage has significantly improved granularity compared with previous AJCC editions. However, clinical usefulness of AJCC to predict survival has lacked consistency. Incorporation of a clinically adequate and relevant metric of radial tumor extent has been particularly difficult. One recently reported strategy has involved measurement of radial tumor extent during pathologic evaluation of the resected specimen. Depth of cholangiocarcinoma invasion has also been shown to correlate with disease-specific and long-term survival and may be incorporated into a future AJCC staging system. However, this technique cannot be used during preoperative evaluation and requires standardization of pathologic practice.
An alternative clinical staging system proposed by the Memorial Sloan Kettering Cancer Center (MSKCC) group incorporates clinical factors directly related to local tumor extent, including presence or absence of PV invasion and presence or absence of lobar atrophy. The AJCC seventh edition T stage and MSKCC T stage are compared in Table 1 . Recent data support the clinical application of the MSKCC staging in helping to predict resectability; in addition, early MSKCC stage (ie, T stage 1) has been associated with improved patient survival when compared with advanced stages. A new staging system with goals of standardizing reporting and addressing all aspects of perioperative care, including resectability, indications for transplantation, and prognosis, has been proposed; clinical application and validation of this system are yet to be published.
| AJCC | MSKCC | |
|---|---|---|
| T1 | Confined to the bile duct | Involves biliary confluence; ± unilateral extension to second-order biliary radicals |
| T2 | Invades beyond the wall of the bile duct to adjacent adipose (a) or hepatic parenchyma (b) | T1 ± ipsilateral PV involvement ± ipsilateral hepatic lobar atrophy |
| T3 | Invades unilateral branches of PV or HA | Involves biliary confluence with bilateral extension to second-order radicles; or unilateral extension to second-order radicles with contralateral PV involvement or contralateral lobar atrophy; or main or bilateral PV involvement |
| T4 | Invades main PV or its branches bilaterally; or CHA; or second-order biliary radicles bilaterally; or unilateral second-order biliary radicles with contralateral PV or HA | — |
Preoperative patient selection and diagnostic techniques
Operative treatment of hilar cholangiocarcinoma continues to pose significant risk. Perioperative mortality ranges from 5% to 10%, and morbidity ranges from 30% to 60%. Only patients with clinical performance status of more than 50% of normal and without significant systemic comorbid disease (Eastern Cooperative Oncology Group grade ≤2) should be considered for curative resection or neoadjuvant therapy and liver transplantation.
Diagnosis of hilar cholangiocarcinoma can be elusive. However, before considering definitive treatment, diagnosis requires presence of a malignant appearing stricture and at least 1 of the following: (1) endoluminal biopsy or cytology positive for cholangiocarcinoma; (2) polysomy by fluorescent in situ hybridization; (3) mass lesion on cross-sectional imaging at the location of the malignant appearing stricture; or (4) CA 19-9 greater than 100. Resection, but not transplantation, can be performed based on malignant appearing stricture alone, on an individual case-by-case basis.
Almost all patients with hilar cholangiocarcinoma are initially evaluated for jaundice. Initial imaging classically reveals intrahepatic, but not extrahepatic, biliary dilation. Biliary anatomy is evaluated with cholangiography. Cross-sectional imaging (either computed tomography [CT] or magnetic resonance [MR] imaging) and biliary imaging (endoscopic retrograde cholangiography [ERC] or MR cholangiography [MRC] or CT cholangiography) should be performed early during the evaluation process. Choice of imaging modality varies with the clinician’s preference and institutional experience. The goals of cross-sectional imaging are to evaluate for local, regional, and distal extent of disease. The 4 specific factors that must be assessed by preoperative imaging are: (1) metastatic disease, (2) ductal involvement, (3) vascular involvement, and (4) extent of hepatic atrophy. Positron emission tomography (PET) is not routinely used during diagnosis and staging of hilar cholangiocarcinoma. Sensitivity for the detection of regional and distant metastases is low. In addition, PET lacks specificity for evaluation of local tumor (particularly sclerosing variant) and nodal metastases.
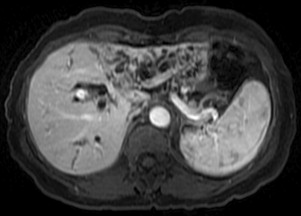
Distant metastases must be excluded before consideration of operative treatment. Nodal basins also need to be evaluated. Lymph node involvement in the regional nodal basin (hepatoduodenal ligament lymph nodes = N1) does not preclude resection but does preclude transplantation. Distant lymph node involvement (celiac or aortocaval nodes = N2) precludes both resection and transplantation. Local extent of disease should be ascertained both directly and indirectly. Cross-sectional imaging with vascular-timed contrast and cholangiography frequently identifies ductal involvement as well as tumor relationship to major vascular structures. Ipsilateral vascular involvement (either PV or hepatic artery) does not preclude resection; contralateral vascular involvement precludes resection but does not preclude transplantation. In cases in which a hilar mass cannot be identified, indirect signs of tumor extent such as vascular (arterial or venous) involvement or lobar atrophy can be ascertained. Evidence of lobar atrophy ( Fig. 2 ) must prompt a careful examination of ipsilateral vascular inflow, in particular PV. On occasion, lobar atrophy can develop from biliary obstruction alone, without vascular compromise.
Current cross-sectional techniques permit concomitant cholangiography either with MRC or CT (CT cholangiography and three-dimensional reconstruction) modalities. Clear definition of the intrahepatic extension of cholangiocarcinoma is critical to resectability and operative approach. Such noninvasive cholangiography concomitantly with contrast-enhanced MR or CT angiography has excellent diagnostic and clinical efficacy. Despite noninvasive diagnostic techniques, most patients require an ERC to obtain an intraluminal specimen for histology or cytology and to provide preoperative biliary decompression. Patients with suspected hilar cholangiocarcinoma require an experienced endoscopist. Percutaneous transhepatic cholangiography (PTC) is reserved for patients who have an inadequate ERC (usually inadequate imaging to determine the extent of left or right duct involvement) or are not amenable to biliary decompression with endoscopy. PTC should be avoided whenever possible. Transperitoneal biopsy or fine-needle aspiration (FNA) of cholangiocarcinoma can lead to tumor seeding and has been associated with higher rates of postoperative recurrence.
Imaging studies should be carefully interpreted by a multidisciplinary team. Individual images cannot be interpreted in isolation and a combination of cross-sectional images and dynamic biliary reconstructions is needed to appreciate tumor presence, location, and vascular involvement. Biliary stricture and corresponding proximal biliary dilatation can help delineate longitudinal extension of tumor along the duct. Viewing of both axial and coronal reconstructions helps to visualize the anatomic relationships between the tumor and vascular structures.
Resection and transplantation criteria
The following criteria for unresectability have been proposed:
- 1.
Distant metastases
- 2.
Lymph node metastases beyond hepatoduodenal ligament (ie, N2 lymph node involvement)
- 3.
Bilateral ductal extension to the secondary (or segmental) biliary radicles
- 4.
Encasement or occlusion of the main PV (or common hepatic artery) proximal to its bifurcation
- 5.
Unilateral involvement of secondary (or segmental) biliary radicles with contralateral vascular involvement
- 6.
Lobar atrophy with involvement of contralateral secondary (or segmental) biliary radicles
- 7.
Lobar atrophy with involvement of contralateral PV or hepatic artery
Patients with unresectability criteria 3 to 7 are candidates for neoadjuvant chemoradiation and liver transplantation, if they have early stage (AJCC stage I or II) lymph node–negative disease. Presence of distant metastases or any nodal metastases precludes liver transplantation.
Liver transplantation inclusion and exclusion criteria are designed to select those patients who are (1) unresectable, (2) least likely to develop metastatic disease, (3) most likely to respond to neoadjuvant chemoradiation, and (4) have the highest probability of survival after liver transplantation. Liver resection and transplantation for hilar cholangiocarcinoma are mutually exclusive therapeutic pathways, without possibility for crossover. Patients found to have unresectable disease during exploration for resection do not do well with subsequent neoadjuvant therapy and liver transplantation. In our experience, operative exploration and subsequent neoadjuvant therapy increase the likelihood for recurrence after transplantation and technical difficulty with transplantation. Conversely, patients who fall out of the neoadjuvant therapy transplantation protocol cannot undergo liver resection even if they are believed to have potentially resectable disease. Neoadjuvant therapy causes widespread hilar biliary necrosis that would make resection and subsequent biliary reconstruction hazardous.
All patients included in the transplant protocol require a diagnosis of hilar cholangiocarcinoma based on presence of malignant appearing stricture and at least 1 of the 4 diagnostic criteria listed previously. In addition, patients with PSC and cholangiocarcinoma are considered to have a field defect and are probably best treated by neoadjuvant therapy and liver transplantation rather than resection, even if they have otherwise potentially resectable tumors.
Candidates for transplantation should not have medical conditions that preclude transplantation and must not have active/uncontrolled infections. In addition, the following criteria preclude neoadjuvant therapy and transplantation :
- 1.
Intrahepatic, gallbladder, or distal cholangiocarcinoma (below the level of the cystic duct)
- 2.
Primary tumor greater than 3 cm in radial diameter (perpendicular to the duct)
- 3.
Any nodal or distant metastases
- 4.
Any surgical attempt at exploration for resection or transperitoneal tumor biopsy or FNA, including endoscopic ultrasound-directed aspiration (EUS) of the tumor. Conversely, EUS/FNA of suspicious regional lymph nodes should be performed to exclude nodal metastases
- 5.
Previous treatment with radiotherapy or chemotherapy that precludes full-dose neoadjuvant therapy
- 6.
History of other malignancy within 5 years
Preoperative patient preparation
Resection
Cholangitis and any organ dysfunction must be addressed before resection of hilar cholangiocarcinoma, if possible. Coagulopathy should be corrected with vitamin K. Systemic antibiotics and biliary drainage should be used before resection and reconstruction of biliary system to minimize perioperative risk of biliary sepsis and liver dysfunction. Biliary drainage of hepatic remnant is usually performed endoscopically when feasible and percutaneously among patients in whom endoscopic decompression cannot be achieved. Preoperative biliary drainage is particularly relevant for the remnant liver when preoperative contralateral PVE is required. Although there has been debate about the usefulness of biliary drainage overall, drainage is appropriate in patients with marked preoperative jaundice or cholangitis. Current data do not support historically discussed risks of increased postoperative infections or tumor seeding with drainage alone; biliary drainage of hepatic remnant should be performed in symptomatic patients before resection. However, drainage of the atrophic liver lobe is contraindicated because of the risk of persistent biliary infection if the tumor proves to be unresectable.
Choice of the extent of hepatic resection is highly dependent on institutional experience. Several high-volume centers routinely perform anatomic hemihepatectomy of the ipsilateral liver in conjunction with the bile duct resection and reconstruction. However, others advocate for an extended hepatectomy to facilitate adequate resection. Margin-negative resection is critical to achieving improved survival. Rates of margin-negative resection or survival have not differed between centers performing standard versus extended hepatectomy. Among patients selected for extended hepatectomy, PVE is frequently needed to optimize the functional liver remnant. Combination of preoperative biliary drainage with PVE augments hepatic hypertrophy in preparation for resection.
Transplantation
Neoadjuvant protocols differ by institution; however, in general, they consist of consecutive external-beam radiation (40–45 Gy) with concomitant 5-fluorouracil chemosensitization, followed by transcatheter radiation with iridium wires (brachytherapy 20–30 Gy). Brachytherapy wires are placed endoscopically, when possible, and via PTC, when an ERC approach fails. After brachytherapy, maintenance capecitabine is administered while patients await transplantation.
The neoadjuvant protocol provides aggressive systemic and locoregional therapy and can result in significant toxicity. Recurrent cholangitis occurs in most, if not all, patients with various degrees of severity. Many patients, particularly those with PSC, can develop hepatic abscesses, requiring preoperative percutaneous drainage and prolonged antibiotic therapy. The patient dropout rate, largely because of tumor progression, after starting neoadjuvant therapy is approximately 11.5% per 3 months, and approaches 30% at 12 months among patients awaiting transplantation. Independent factors associated with dropout from neoadjuvant protocol are initial CA 19-9 500 or greater, tumor 3 cm or greater, and MELD (Model for End-Stage Liver Disease) 20 or greater. Routine pretransplant surveillance is performed every 3 months before transplantation as required by United Network for Organ Sharing/Organ Procurement and Transplantation Network policy for patients with malignancy awaiting liver transplantation. Evidence of disease progression or metastases while on the neoadjuvant protocol precludes transplantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



