© Springer International Publishing Switzerland 2016
Celestino Pio Lombardi and Rocco Bellantone (eds.)Minimally Invasive Therapies for Endocrine Neck Diseases10.1007/978-3-319-20065-1_88. High-Intensity Focused Ultrasound Ablation (HI-FU) in Endocrine Neck Diseases
(1)
Department of General Surgery, Hopital de la Pitié, Paris 6 University, 83 boulevard de l’hopital, Paris, F-75013, France
(2)
Department of Nuclear Medicine, Hopital de la Pitié, Paris 6 University, Paris, F-75013, France
(3)
ENT, 6 Rue Puvis de Chavannes, Paris, F-75017, France
Thyroid nodules and primary hyperparathyroidism (HPT) are highly prevalent endocrine disorders. Fortunately, almost 100 % of parathyroid tumors and more than 95 % of thyroid nodules are benign. Some patients with thyroid nodules will need surgery for diagnostic purpose despite the improved accuracy of noninvasive procedures, and others for HPT. Complications of thyroid and parathyroid surgery such as recurrent laryngeal nerve (RLN) injury, superior laryngeal nerve injury, hypocalcaemia after total thyroid resection, and hemorrhage are not so rare and permanent complications can occur, respectively, at 2.1 % and 2.7 % RLN palsy, and hypoparathyroidism rates have been reported in a prospective multicenter study including departments specialized in endocrine surgery [1].
Moreover, some patients in poor medical condition are contraindicated for a surgical neck exploration for a local recurrence of thyroid carcinoma or for a parathyroid adenoma because of a high risk of surgery and/or general anesthesia.
This is why minimally invasive or noninvasive methods have been developed to ablate thyroid nodules and parathyroid glands without surgery: percutaneous ethanol injection therapy, percutaneous thermal procedures (radiofrequency ablation, laser coagulation), microwave ablation, and high-intensity focused ultrasound (HIFU) [2].
HIFU has been studied for 50 years, with recent technological developments allowing its use for tumors of various sites. This technique was initially employed to treat localized prostate cancer [3] and is currently widely used in clinical practice in this indication. A recent review showed that localized prostate cancer could be eradicated by HIFU with a reduction of associated side effects of radical treatment, although long-term follow-up studies are still needed to evaluate cancer-specific and overall survival [4]. In the field of prostate cancer, HIFU has also been described among salvage local therapies after failure of radiotherapy [5].
For other tumor locations, clinical applications are still to be established [6]. With the exception of prostate cancer, literature is relatively poor. However, recent technological developments allow its use for tumors of the liver to treat unresectable advanced stages of hepatocellular carcinoma and liver metastases [7].
Concerning HIFU ablation of thyroid nodules or parathyroid glands, there is no randomized cohort studies. The only reported experience is limited to studies from two teams using this procedure to treat thyroid nodules [8] or HPT [9].
The first experience of HIFU for localized thyroid tissue ablation in an animal was published only 10 years ago by Esnault O. et al. [8]. This preliminary study confirmed the possibility of using HIFU for ablation of a defined area in thyroid tissue in an experimental model performed in ewes with a device not fully suitable for this purpose (it was a device for prostate cancer treatment). An additional study was performed on ewes to evaluate the reproducibility of a HIFU prototype designed specifically for human use [10]. First human feasibility studies are very recent since HIFU ablation of thyroid nodules was published only in 2011 for 25 patients included from 2003 to 2006 [11], and HIFU ablation of parathyroid adenoma was only reported in 2010 (4 patients, date of inclusion unknown) [9].
8.1 Technique
The originality of HIFU ablation is that this procedure induces thermal destruction without any skin penetration. It delivers a large amount of heat energy to a restricted space, where ultrasound (US) produces necrosis with a minimum effect on surrounding structures. Energy deposit caused by the interaction between high-intensity US and the tissue will raise the temperature locally in the target area [6].
Ultrasound guidance is used to position the device accurately over the right or the left lobe of the thyroid: an extracorporeal probe (3 MHz frequency) can locate the zone to be treated during the session and trachea, esophagus, and other parts potentially at risk such as the carotid are carefully avoided.
Two parameters must be defined: acoustic intensity and focus of the US beam. Acoustic intensity is usually set at around 100 W/cm2. The focus of US beam is activated by using multiple, convergent, and specific transducers, creating a concentration of energy at a focal point and producing the ablative effect. Focus within the tissue is highly collimated and allows an energy concentration that is followed by an increased temperature within the focal volume, resulting in tissue coagulative necrosis. Multiple impulses are needed to induce an ablation volume of clinical significance. An effective treatment area of 2 mm in diameter by 5 to 8 mm in depth is obtained for each sequence of US impulses, which generally lasts about 5 s. For each treatment cycle, around 100 J of energy are delivered, heating up the focal point, the objective being to exceed 70–75 °C locally in order to destroy the target cells. This method is extremely accurate since energy drops sharply outside the focal zone: tissue 0.3 mm away from the point focal remains unchanged [12].
The procedure is usually performed under conscious sedation, can be employed in an ambulatory setting, and requires from 1 h to a few hours to achieve an effective volume ablation [11]. Local anesthesia is administered on the skin, and in some cases, inside the nodule itself. Not every thyroid nodule can be treated all at once, but experience in prostate disease shows that a 40 g prostate can be entirely treated in one session [13]. For thyroid nodules, HIFU can be repeated if the first treatment was incomplete: it was the case for 2 patients (out of 25 in the clinical study) who had a second HIFU session on day 8 for no significant reduction in vascularization in the treated nodule by Doppler ultrasonography [11].
8.2 Indications
Clinical experience is limited and no study was performed to compare HIFU ablation with other noninvasive or minimally invasive alternative treatments such as ethanol injection therapy, radiofrequency ablation, or laser coagulation [14].
As with diagnostic US, sound waves do not pass through air or solid structures, like bone. This prevents the treatment of tumors behind the sternum (substernal goiter and mediastinal parathyroid adenoma).
8.2.1 Thyroid Disease
8.2.1.1 Euthyroid Nodular Goiters
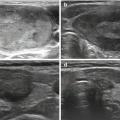
HIFU ablation could be used to treat benign or malignant lesions of the thyroid without having to resort to surgery and without disrupting any subsequent treatment. However, the only clinical data on HIFU ablation for the thyroid gland nodules come from a single research center [11, 14, 15]. In an open feasibility study, 25 patients were treated with HIFU two weeks before surgery for nodular goiter [11]. Treatment was disrupted in 3 patients. Among the remaining 22 patients, macroscopic and histological examinations showed that all lesions were confined to the targeted nodule without affecting adjacent structures. Although 17 out of the 22 treated patients showed no US change in nodule size at 15 days, an extent of nodule destruction (ranged from 2 to 80 %) was observed on pathology in every patient, including 5 patients who had over 20 % pathological lesions unmistakably attributed to HIFU: Typical lesions were coagulative necrosis, multiple fibrotic scars, thrombosis, surrounding a cystic area; less specific lesions associated with ultrasound changes were noncoagulative necrosis, hemorrhage, nodule detachment, cavitations, and cysts). The last three patients ablated at a highest energy level showed a significant ultrasound change and complete coagulative necrosis in 80 %, 78 %, and 58 % of the targeted area, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree