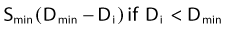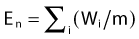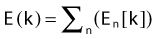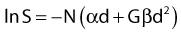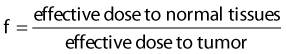14 High Dose Rate Brachytherapy
Remote controlled brachytherapy can be performed using low dose rate (LDR), medium dose rate (MDR), or high dose rate (HDR) techniques. Although the International Commission on Radiation Units and Measurements (ICRU) #38 definition of HDR is >12 Gy/hr,1 the usual dose rate employed in current HDR brachytherapy units is about 100 to 300 Gy per hour. HDR has the added advantage that the treatments take only a few minutes, which can be given on an outpatient basis with minimal risk of applicator movement and minimal patient discomfort. Additionally, use of a single-stepping source, as used in most modern HDR afterloaders, allows optimization of dose distribution by varying the dwell time at each dwell position. However, it should be emphasized that, although, optimization can improve the dose distribution, it should not be used to substitute for a poorly placed implant. Nag and Samsami2 have provided examples of inappropriate optimization strategies which can lead to suboptimal dosimetry plans and clinical problems. HDR is normally given as a course of a number of fractionated HDR treatments, although it can be given as a single treatment, as in intraoperative HDR brachytherapy, if doses to the normal tissues can be sufficiently reduced by displacement or shielding. The advantages and disadvantages of HDR in comparison to LDR are enumerated in Table 14-1.
Table 14-1 Advantages and Disadvantages of High Dose Rate Compared With Low Dose Rate Brachytherapy
| Advantages | Disadvantages |
|---|---|
| 1. Radiation protection HDR eliminates radiation exposure hazard for caregivers and visitors. Caregivers are able to provide optimal patient care without fear of radiation exposure. HDR eliminates source preparation and transportation. Since there is only one source, there is minimal risk of losing a radioactive source. | 1. Radiobiological The short treatment times do not allow for the repair of sublethal damage in normal tissue, or the redistribution of cells within the cell cycle or reoxygenation of the tumor cells; hence, multiple treatments are required. |
| 2. Allows shorter treatment times There is less patient discomfort since prolonged bed rest is eliminated. It is possible to treat patients who may not tolerate long periods of isolation and those who are at high risk for pulmonary embolism due to prolonged bed rest. There is less risk of applicator movement during therapy. There are reduced hospitalization costs since outpatient therapy is possible. HDR may allow greater displacement of nearby normal tissues (by packing or retraction) which could potentially reduce morbidity. It is possible to treat a larger number of patients in institutions that have a high volume of brachytherapy patients but insufficient inpatient facilities (e.g., in some developing countries). Allow intraoperative treatments, which are completed while patient is still in the operating room. | 2. Limited experience Few centers in the United States have long-term (>20 years) experience. Until recently, standardized treatment guidelines were not available; however, the American Brachytherapy Society (ABS) has recently provided guidelines for HDR at various sites.121,122,128,130,135,147,185 |
| 3. HDR sources are of smaller diameter than the cesium sources that are used for intracavitary LDR This reduces the need for dilatation of the cervix and therefore reduces the need for heavy sedation or general anesthesia. High-risk patients who are unable to tolerate general anesthesia can be more safely treated. HDR allows for interstitial, intraluminal and percutaneous insertions. | 3. The economic disadvantage The use of HDR brachytherapy compared to manual afterloading techniques requires a large initial capital expenditure since the remote afterloaders cost about $300,000. There are additional costs for a shielded room and personnel costs are higher as the procedures are more labor intensive. |
| 4. HDR makes treatment dose distribution optimization possible Variations of the dwell times of a single stepping source allow an almost infinite variation of the effective source strengths, and the source positions allows for greater control of the dose distribution and potentially less morbidity. | 4. Greater potential risks Since a high activity source is used, there is greater potential harm if the machine malfunctions or if there is a calculation error. The short treatment times, compared to LDR, allow much less time to detect and correct errors. |
HDR, High dose rate; LDR, low dose rate.
Physics of High Dose Rate Brachytherapy
Dose Optimization for High Dose Rate Brachytherapy
Significant technological improvements have been made in imaging devices, and better quality images are now obtained. More details of the anatomy can be seen, sharper organ boundaries can be delimited, and with the development of functional imaging3; it is often possible to see tumors within organs. In parallel to modern imaging modalities, computer speed and capacity have experienced exponential growth. Visually impressive planning programs are available off the shelf and can be used to calculate dose distributions within minutes.
Inverse Planning Dose Optimization
Few algorithms that specifically use the anatomy (target and organs at risk, as well) to guide the optimization have been developed. Tumor shape and the relative positions between the organs at risk and the tumors and the intent of the clinicians all contribute to define the objectives and dose constraints of the optimization engine. One approach is to obtain an optimized plan for each set of constraints and present all the possible solutions to the planner. Then, the planner can use his or her experience or a decision-making tool to select the solution that provides the best fit for the clinical goals.4 Alternatively, one can combine the conflicting constraints as a weighted sum.5–7 This way, coverage, conformity, and uniformity of the dose distribution must be balanced for all volumes of interest, targets, and organs at risk alike. Weight factors or importance factors are used to prioritize the dose constraints. This approach, called inverse planning, has been successfully developed and implemented at the University of California at San Francisco.8–9
The minimization of the adaptable objective function requires an optimization technique that allows for wider sampling and hill climbing to escape from a local minimum. This is because of the fact that the cost function E(k) is mathematically nonlinear and therefore presents multiple minimums. Simulated annealing (SA), first introduced by Kirkpatrick,10 and other related algorithms (Fast SA, Very Fast SA) are optimization techniques that can process cost functions with arbitrary boundary conditions with the statistical guarantee of finding an optimal solution. Equally important, SA class algorithms are easily coded compared to other nonlinear optimization algorithms. It has been shown that the fast simulated annealing algorithm can be used to govern an optimization process to automatically and rapidly produce a plan for prostate permanent implant6,11 treatments, as well as for afterloading HDR prostate treatments.12
The inverse planning tool is now widely available. It was used for dose planning of several thousand patients patients8–9,12–23 with anatomic sites including prostate, gynecologic (vaginal cylinders, tandem-ovoids, and interstitial templates), rectum, sarcomas, base of tongue, and nasopharynx. In prostate treatment, the urethra can be considered a second target, because it requires the full dose but also requires protection from high dose spots. One can also include other COs such as the bulb of the penis14 and specifically protect the neurovascular bundles or boost a dominant intraprostatic lesion.
Several centers have compared the use of inverse planning with their own previous planning approaches, including geometrical optimization,8 graphical optimization,21,23 and dose point optimization.23 All those centers report a significant dosimetric advantage over other optimization techniques. The advantages include improved dose coverage, comformality and homogeneity of the target, a significant reduction of the urethra dose and of the normal tissue, as well as a reduction of the planning time.
The concept of prescribing at a given point no longer applies for inverse planning; the prescription is global. The approach of inverse planning allows the increase of the number of organs at risk with specific dose constraints without adding to the complexity of the problem. In the case of the treatment of prostate, for instance, the dose received by the bulb of the penis can be significantly reduced by including it as an additional organ at risk. It was shown that on average, the volume of the bulb receiving 75% of the prescribed dose is reduced by 80% for a small penalty of 2% on the target coverage.15
Clinical Implementation
Since CT or MRI contours of the CTV and CO are used not only to define the anatomy, but also to constrain the dose distribution, they provide the clinician with added flexibility and control to shape the dose distribution. The availability of inverse planning tools allows the planning of complex dose distributions that were not considered before. New organs at risk, not taken into account before, such as the bulb of the penis14 or the neurovascular bundles, will become an integral part of the dose constraints.
As we move from the two-dimensional (2D) brachytherapy planning system to the new 3D era, the necessity of an inverse planning algorithm becomes clear. More details can be found in Chapter 13. With hundreds of active dwell positions, irregularly shaped volumes, multiple target and organ volumes, and multiple organ sensitivities, the chance of finding dwell times that would optimally satisfy all the requirements manually becomes more and more remote. Armed with the next-generation brachytherapy planning system, systematic evaluation of partial organ tolerance on a finer scale will become possible. This understanding of partial organ tolerance will be the key to the next generation of brachytherapy.
Radiobiology of High Dose Rate Brachytherapy
Repair of Sublethal Damage
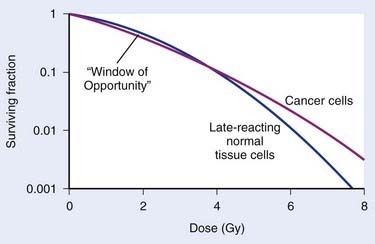
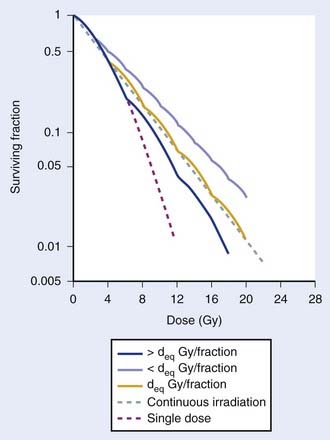
The major radiobiological difference between LDR and HDR brachytherapy relates to repair of sublethal damage. This is vitally important, because it is the differences in repair between tumor and late-responding normal tissue cells that make radiotherapy a viable way to treat cancer. These differences in repair cause the shapes of survival curves to differ, as illustrated in Fig. 14-1. Survival curves for some tumor cells tend to be flatter (more linear) and have a greater initial negative slope than those for late-reacting normal tissue cells.24 Inasmuch as survival curves for late-reacting normal tissue cells are “curvier,” they exhibit a larger shoulder at low doses which represents a greater potential for repair of sublethal damage. A fractionated course of radiotherapy capitalizes on this concept. Using multiple fractions of radiation, each of small size, allows more repair of the normal tissue compared with that of some cancer cells. There is essentially a “window of opportunity” whereby treatment at dose/fraction below the crossover point of the two survival curves in Fig. 14-1 results in more killing of tumor than normal tissue cells, and this is the reason we fractionate. It should be noted that this crossover point will be highly tumor- and normal tissue-specific, which is why the optimal dose/fraction is not the same for all clinical situations.
Because repair takes time, with typical half-times for repair on the order of 1 hour,25–29 it is generally agreed that at least 6 hours should be allowed between fractions if this repair advantage of fractionation is to be fully realized. Alternatively, irradiating continuously at LDR allows sufficient time during the treatment for repair to occur. Then the major radiobiologic difference between HDR and LDR is that the repair advantage with HDR is realized by using many low-dose fractions separated by enough time for most repair to occur between fractions; with LDR, the repair advantage is realized by using a dose rate low enough to allow repair during the irradiation. Replacing LDR with HDR necessitates the simulation of the dose-rate effect of LDR by the fractionation effect of HDR. This is illustrated by the cell survival curves shown in Fig. 14-2.30 The dashed line represents the single-dose survival curve at high dose rate (too high to allow any repair during the irradiation). With continuous LDR irradiation, the curve (dotted line) moves to the right (more survival) due to increased repair, and straightens out, since most repairable damage will be repaired, leaving only irreparable damage, which varies linearly with dose.31 This is the so-called dose-rate effect.31 Selecting the HDR dose/fraction that generates a fractionated survival curve that falls exactly along this continuous irradiation line achieves equivalency between the HDR and LDR regimens. This is shown by the deq Gy/fraction “wavy” line in Fig. 14-2. The “waviness” of this survival curve is a result of the repetitive shoulder for each fraction, which represents repair between successive fractions. Treating at a low dose/fraction (<deq Gy/fraction) will result in more repair and hence a shallower survival curve, as shown by the upper curve in Fig. 14-2. Conversely, treating at a higher dose/fraction (>deq Gy/fraction) causes the survival curve to become steeper.
Although Fig. 14-2 illustrates how survival curves for HDR and LDR can be similar, it does not address the important effect of the differences in shapes of the survival curves of cancer and late-responding normal tissue cells shown in Fig. 14-1. Complete equivalence of HDR and LDR regimens requires that the HDR and LDR survival curves be coincident for both tumor and normal tissue cells. Although not obvious from Fig. 14-2, it can be shown that it is theoretically possible to find a value of deq for which this will occur.26 Demonstrating this requires the use of a mathematical model that represents the shapes of these survival curves. Dale27 did just this using the linear-quadratic (L-Q) model for cell survival.
The Linear Quadratic Model
The L-Q equation for cell surviving fraction, S, is:
where d is the dose/fraction (of HDR or LDR), N is the number of fractions, α and β are parameters that define the “curviness” of the survival curves, and G is a function that accounts for the repair that occurs during and between fractions.27,32–33 Clearly, G depends on dose rate, dose/fraction, time between fractions, and the rate of repair. Dale27 published equations to account for changes in all these variables.
Using Dale’s equations, it has been shown that HDR surviving fractions can exactly match those with LDR for both cancer and normal tissue cells, with a reasonable number of HDR fractions, if it is assumed that tumor cells repair faster than normal cells.34 For example, if the half-times for repair are assumed to be 0.5 hour for tumor and 1.5 hours for late-reacting normal tissue cells,25,26,28,29 a course of 60 Gy at LDR can be matched by a 7-fraction HDR regimen at 6 Gy/fraction, using representative L-Q model α and β parameters. This is illustrated in Fig. 14-3.
Note that in Figs. 14-1 and 14-3, it has been assumed that the dose to normal tissues is the same as the tumor dose. However, a major advantage of brachytherapy is that this is a highly conformal type of radiotherapy, so the important physical advantage of brachytherapy has been neglected. This can be accounted for in L-Q model calculations by the use of the geometrical sparing factor.35
Geometrical Sparing Factor
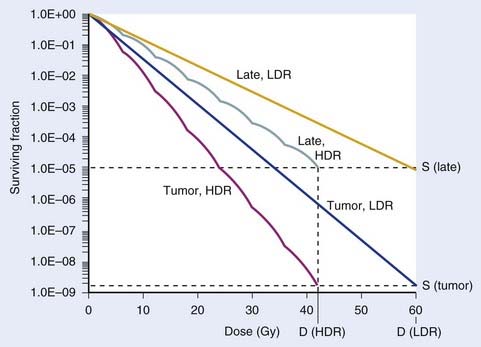
Since brachytherapy dose distributions are significantly inhomogeneous, it is not readily obvious how to compare tumor and normal tissue doses. One way to do this is to reduce these dose distributions to “effective” doses, where the effective dose is that which, if delivered uniformly to the tumor or normal tissue, exhibits the same local control or risk of injury, respectively, as the inhomogeneous distribution it depicts.33 Then the geometrical sparing factor (f) can be defined as:
which, for brachytherapy, should normally be less than unity. This can have a profound effect on the comparison of tumor and normal tissue cell survival curves, as illustrated in Fig. 14-4, in which the tumor curve and the late-responding normal tissue curve for f = 1 (dashed) are the same as the two curves shown in Fig. 14-1, although taken out to higher doses. If, as illustrated, the dose axis represents the tumor dose, and a modest geometrical sparing factor of 0.8 is assumed, the normal tissue curve shifts 20% to the right and the crossover point moves all the way out to about 13 Gy. The “window of opportunity” now opens considerably, giving a much wider range of doses over which the repair advantage of normal tissues can be exploited. For example, using the L-Q model, one can show that with f = 0.8, the number of HDR fractions required to be equivalent to LDR (shown in Fig. 14-3) reduces from 7 to 6, and with f = 0.6, this number reduces to only 4. Hence, a modest improvement in geometrical dose sparing makes use of an HDR program practical.35–36
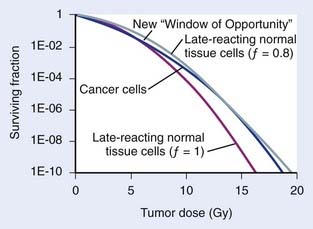
FIGURE 14-4 • Effect of geometrical sparing of normal tissues on the crossover point for tumor and normal tissue survival curves. With the L-Q model parameters used here, the crossover point moves from about 5 Gy when the geometrical sparing factor (f) = 1 (see Fig. 14-1), out to about 13 Gy when f = 0.8. Note that the “window of opportunity” now extends out to much higher doses (and doses/fraction).
Note that the cell surviving fraction curve for late reacting normal tissue cells at LDR in Fig. 14-3 was plotted using a half-time for repair of 1.5 h. However, Bentzen et al.37 have reported that their study of late reactions with continuous hyperfractionated accelerated radiotherapy (CHART) shows that this half-time for repair might well be much longer than 1.5 h, at least for some normal tissues. It has subsequently been demonstrated that geometrical sparing, combined with potentially slower repair of late-reacting normal tissue cells, could even make HDR superior to LDR.38 With this radiobiological model, the resultant calculations provide only a fair representation of the highly complex biological changes that occur in tissues exposed to a course of brachytherapy. For example, reoxygenation, repopulation, and the inverse dose-rate effect have been neglected. Nevertheless, analysis of results of several decades of experience with tens of thousands of patients treated for cervical cancer shows that replacing LDR with an HDR regimen that has four to seven fractions39 is feasible, and affirms the concordance of theory and clinical practice. In summary, radiobiological modeling shows that it is possible to replace LDR brachytherapy with HDR without a decrease in the probability of tumor control or an increased risk of complications. The number of fractions necessary to achieve equivalency between HDR and LDR regimens is multifactorial and depends on the radiobiologic properties of the irradiated tissues (e.g., the relative half-times of repair and α/β values assumed for tumor and normal-tissue cells) and the physical “geometrical sparing” of the normal tissues. Commonly used HDR regimes with four to seven fractions seem to satisfy these requirements.
Practical Use of Radiobiological Modelling for Clinicians
Fractionation schemes for HDR are widely variable, and many radiation oncologists are not very familiar with the resultant biological effects. Empirical methods such as the NSD (nominal standard dose), TDF (time-dose factor), or a dose reduction factor of 0.6 have been used in the past to convert HDR doses to LDR equivalent doses. The L-Q equation given above can be used to guide development of HDR doses and fractionation schedules. However, the L-Q mathematical calculations are tedious and may not be practical on a day-to-day basis. Hence, a simplified computer program was developed by Nag and Gupta40 to obtain the isoeffective doses to be used for HDR. The clinician needs only to enter the EBRT total dose and dose/fraction, HDR dose, and the number of HDR fractions. The program will automatically calculate the isoeffective doses for tumor and normal tissue effects. Isoeffective doses are expressed in clinically familiar terms (as if given at 2 Gy per fraction) rather than as biologically equivalent doses (BED), which are unfamiliar to clinicians. Furthermore, a dose-modifying factor (DMF) is applied to the normal tissues to account for the fact that doses to normal tissues are different from the doses to the tumor, thus, providing a more realistic equivalent normal tissue effect. This program can therefore be used to determine isoeffective HDR doses to treat various cancers. Alternatively, the program may be used to express the isoeffective dose of different HDR dose-fractionation regimes.
In view of the many limitations of the L-Q model, it must be stressed that, as with any mathematical model, the L-Q model should be used judiciously as a guide only and should always be correlated with clinical judgment and outcome results. Caution is especially warranted whenever large fraction sizes are used, since their clinical results have not been well studied.35
Clinical Uses of High Dose Rate Brachytherapy
Carcinoma of the Cervix
Role of High Dose Rate Brachytherapy
Brachytherapy is an integral part in the definitive management of cervical cancer. Traditionally, LDR brachytherapy has been used in conjunction with EBRT. More recently, HDR brachytherapy has become increasingly popular in the United States because of practical and patient care advantages. These advantages include therapy delivered solely on an outpatient basis, avoidance of long-term bed rest with its associated increased risk of deep venous thrombosis and pulmonary embolus, and avoidance of significant cervical dilation.41 Furthermore, potentially improved dose optimization to tumor exists while simultaneously sparing normal tissues (i.e., bladder and rectum) with the ability to adjust individual dwell times, and improved confidence in constant tumor coverage with more rapid delivery of treatment resulting in less risk of applicator displacement during treatment. Finally, integration of HDR brachytherapy during the course of EBRT is possible.42 A disadvantage of HDR brachytherapy for carcinoma of the cervix is the increased number of HDR treatments required to deliver doses that are equivalent to that given by LDR.
Delivery of High Dose Rate Brachytherapy
Uterovaginal Applicator
Applicator Description
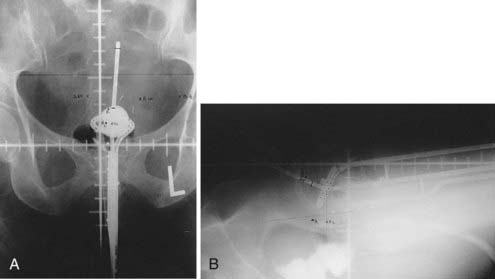
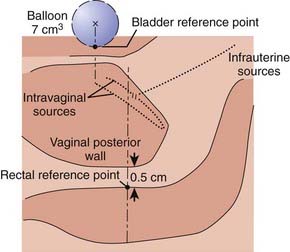
Various uterovaginal applicators are commercially available. The details of these applicators vary depending on the manufacturer. In general, delivery of HDR brachytherapy can be accomplished with the use of either tandem and ovoids (e.g., Fletcher applicator) or tandem and ring applicator. A typical tandem and ring applicator consists of three components that are interconnected: tandem, ring, and rectal retractor (depicted in Fig. 14-5). The tandem is available in three angulations (30°, 45°, and 60°) and three lengths (2, 4, and 6 cm). The caliber of the tandem is narrow and approximates the diameter of a typical uterine sound and thus obviates the need for cervical dilation for placement. The ring is available in three diameters (26, 30, and 34 mm), each with its own plastic cap (3 to 4 mm thick) and corresponding angulation (30°, 45°, and 60°) to match the tandem. An optional rectal retractor has built-in metal markers allowing easy identification of the posterior vaginal mucosa on plain radiographs and thus, facilitating dose calculation for the rectum (Fig. 14-6). These applicators are now available in a CT/MRI–compatible material to allow more accurate 3D conformal planning discussed later. The tandem is first inserted into the cervical canal followed by the ring and then the rectal retractor. The tandem, ring, and rectal retractor are interconnected, thereby ensuring that the tandem is situated at the epicenter of the ring and thus, preventing anterior or posterior displacement of the ring. Further, the fixed geometry system permits the use of preplanned dosimetry for rapid patient treatment, if required, to minimize patient discomfort.
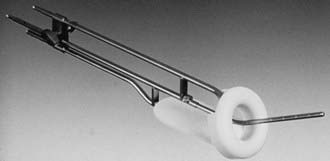
FIGURE 14-5 • Uterovaginal HDR ring applicator.
(Courtesy Nucletron Corporation, Veenendaal, The Netherlands.)
Applicator Placement
Inserting approximately 150 to 200 mL of sterile saline solution into the bladder enhances the resolution of the ultrasound image. Gently wiggling the tandem during the imaging procedure helps to identify the tandem within the uterine cavity. Sagittal and axial images are performed to ensure there is no fundal penetration or creation of false tracts inside the uterine wall. The tip of the tandem is ideally situated within 1.0 to 1.5 cm from the fundal serosa in the normal-sized uterus (<8 cm uterine cavity length using a 6-cm length tandem). In large size uteri, the distance between the tip of the tandem and the fundal serosa may be farther in the initial application, but may decrease as the uterus size decrease with further external irradiation, chemotherapy, and HDR brachytherapy. The application placement is completed with the placement of the vaginal ring or ovoids (typically the largest size that anatomically is acceptable) and bladder and rectal retraction. If a narrowed vaginal vault does not allow optimal positioning of the ring or ovoid, an interstitial technique is preferable. In the event that an interstitial technique is not available, Sharma et al. have described an intracavitary technique which utilizes a central tandem with a single ovoid alternating with the contralateral ovoid during subsequent insertions.43 Another possibility is to use a tandem and cylinder, but this will result in a higher dose to the bladder and rectum.
Dose Prescription Points and Imaged-Based Treatment Planning
Traditionally, the dose for cervical brachytherapy has been prescribed to an applicator-based point, point A, defined as 2 cm cephalad along the axis of the tandem plus an allowance of 3 to 5 mm for thickness of the plastic cap covering and then 2 cm laterally from the axis of the tandem.44,45 Typical plain film computerized dose calculations are illustrated in Fig. 14-7. The specifications of bladder and rectal dose calculations are given in International Commission on Radiation Units and Measurements (ICRU) Report No. 38 and are illustrated in Fig. 14-8.1 The rectal and bladder doses are preferably limited to 75% of the point A dose.
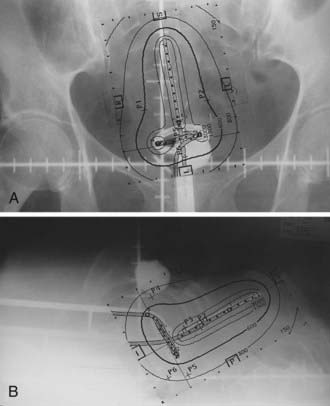
FIGURE 14-7 • Anterior-posterior (A) and lateral (B) isodose distributions of uterovaginal application.
With the advent of CT/MRI–compatible applicators, image-based treatment planning has become more feasible. MRI is superior to other current imaging modalities in delineating gross tumor involving the cervix and adjacent normal tissues.46–48 The underlying principle for using modern radiographic imaging techniques such as MRI is to potentially better delineate the target volume, which could allow dose escalation for better tumor control while simultaneously limiting dose to normal tissues. This contrasts with prescription doses that are referenced to point A, because, unfortunately, it does not necessarily reflect the dose to the tumor.
A gynecological working group was formed by the Groupe Europeen de Curietherapie and European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) in 2000 to formulate and describe new terminology regarding 3D image–based treatment planning in cervical cancer brachytherapy. Their recommendations were published in March 2005 and are summarized in Table 14-2.49 This working group recognized that there is significant change of gross tumor during treatment and thus, a fluid description of treatment volumes during treatment is required to have a better understanding of dose to volume relationships and to more accurately compare treatments between institutions and patients. They devised definitions of gross tumor volume (GTV) at diagnosis and at each brachytherapy procedure as well as high risk and intermediate risk CTVs. Each of these volumes would be determined via clinical examination and MRI (preferably T2 weighted) at their respective times during the course of treatment. The CTVs are based on tumor load and represent risk of recurrence. High risk is that which includes macroscopic tumor, intermediate risk is that which includes significant microscopic disease and low risk is that which includes potential microscopic tumor spread. It is assumed that the low risk region is successfully treated with surgery and/or external beam radiotherapy.
Table 14-2 GEC-ESTRO Image-Based Planning Volume Definitions for Cervical Cancer
| Volume | Description | |
|---|---|---|
| GTVD | GTV at diagnosis | Macroscopic tumor extension at diagnosis as detected by clinical examination and visualized on MRI |
| GTVB1,B2,B3… | GTV at each BT procedure | Macroscopic tumor extension at time of brachytherapy as detected by clinical examination and as visualized on MRI |
| HR CTVB1,B2,B3… | High risk CTV at each BT procedure | Includes GTVB1,B2…, the whole cervix and presumed extracervical tumor extension at time of brachytherapy by means of clinical examination and by MRI |
| IR CTVB1,B2,B3… | Intermediate risk CTV at each BT procedure | Encompasses high risk CTV with a safety margin of 5-15 mm (Safety margin is chosen according to tumor size and location, potential tumor spread, tumor regression, and treatment strategy; the IR CTV is never less than GTVD) |
GTV, Gross tumor volume; CTV, clinical target volume; BT, brachytherapy; MRI, magnetic resonance imaging; GEC-ESTRO, Groupe Europeen de Curietherapie of European Society for Therapeutic Radiology and Oncology.
In early 2006, the working group released an update to their original recommendations to include descriptions of dose volume parameters for organs at risk and a step-wise procedure for transition from the traditional dose prescription to the 3D image–based volume prescription.50 The group recommended that for organs at risk that the minimum dose in the most irradiated tissue volumes of 0.1 cm3, 1 cm3, and 2 cm3 be reported. Ultimately, it is their intention not to alter current clinical and dosimetric reporting, but to assess the feasibility, value, and reproducibility of such volumes.
Dose Fractionation
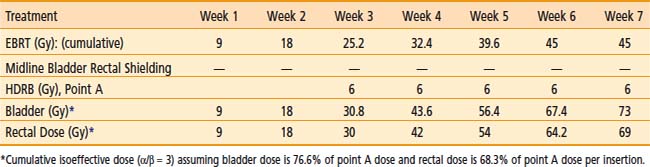
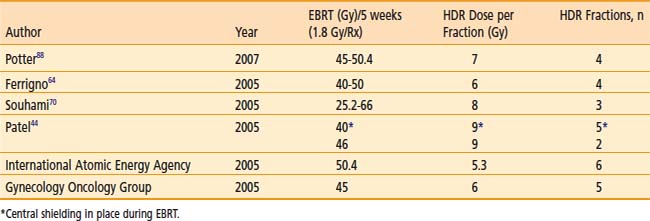
Various dose fractionation schedules have been used in the United States and worldwide (Table 14-3).45,51–54 Most commonly used regimens use fraction sizes between 5 and 7 Gy in four to six fractions, given on a weekly basis.45,55–56
Table 14-3 Carcinoma of the Cervix: Various Combined External Beam Radiation Therapy (EBRT) and High Dose Rate (HDR) Brachytherapy Regimens
When patients are treated with LDR brachytherapy, the intracavitary application is typically performed after the EBRT component to the pelvis. The use of HDR allows integration of brachytherapy earlier into the course of EBRT, typically after two weeks (20 Gy) of EBRT. The earlier integration of HDR in the radiotherapeutic management allows for the total duration of the treatment program to be shorter than eight weeks, as recommended.45 Extended duration of radiation therapy (external and brachytherapy) for cervical carcinoma beyond eight weeks is adversely associated with a lesser control rate and survival. Moreover, the earlier integration of HDR brachytherapy provides an enhanced ability to concentrate dose delivery to the primary tumor and to reduce symptoms such as bleeding. The potential disadvantage of earlier integration of HDR brachytherapy is the reduced dose to the periphery of the tumor in bulky barrel-shaped tumors (when combined simultaneously with the use of a midline bar to shield the bladder and rectum).
The American Brachytherapy Society (ABS) has suggested a number of therapeutic schemas for the integration of EBRT and HDR brachytherapy (Table 14-4).45 In general, a lower central dose contribution from EBRT necessitates more applicator insertions and/or increased HDR fraction size per insertion. Typical treatment scenarios are henceforth described for early stage disease and locally advanced disease. For non-bulky stages I and II (<4 cm primary) EBRT is delivered using a four-field box technique to a dose of 45.0 Gy (1.8 Gy per fraction). Following a dose of 19.8 Gy of EBRT, weekly HDR uterovaginal applications are initiated each delivering 6.0 Gy to point A. The patient would undergo a total of five HDR uterovaginal insertions and EBRT is not performed on the day of HDR brachytherapy (Table 14-5). This combination of EBRT and HDR brachytherapy would equate to a total LDR equivalent dose of approximately 84 Gy to point A.40
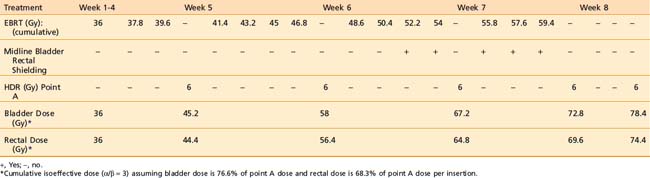
Table 14-4 American Brachytherapy Society Suggested Doses of External Beam Radiation Therapy (EBRT) and High Dose Rate Brachytherapy to be Used in Treating Early and Advanced Cervical Cancer
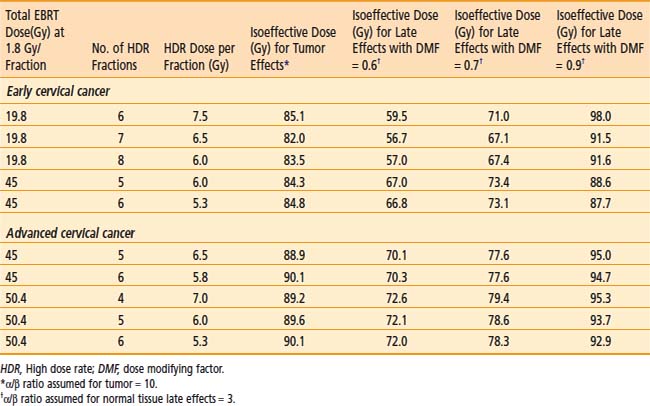
Some institutions place a midline block after 19.8 Gy of EBRT in early cases and increase the HDR doses (either additional HDR fractions or higher HDR dose per fraction). For bulky lesions (stages IB and II ≥4 cm or stages IIIA, IIIB, and IVA) anatomic distortion by the tumor may preclude the insertion of the tandem at 19.8 Gy and therefore EBRT using the four-field technique continues to 39.6 Gy and tandem insertion is reattempted. Whole pelvic EBRT is continued to a total of 45 to 50.4 Gy at which time a midline block is placed and followed by supplemental doses to the involved parametria/sidewalls to a total dose of 54.0 to 59.4 Gy with special attention given to avoiding additional small bowel irradiation. The midline block is designed to block the approximate 6 Gy isodose line. Again, five HDR applications are performed each delivering 6.0 Gy to 6.5 Gy to point A based on the whole pelvic EBRT dose (Table 14-6). With better imaging and dosimetry techniques, many centers are now using fewer fractions while increasing the dose per fraction (e.g., four fractions of 7 Gy or three fractions of 8 Gy) to obtain isoeffective doses.
Results of LDR versus HDR
The majority of published reports comparing HDR to LDR in the treatment of carcinoma of the cervix have been from nonrandomized studies, although a few randomized studies have been reported. Early studies using HDR brachytherapy demonstrated good local control and survival rates compared to LDR but with increased late small bowel complications, particularly when the HDR dose exceeded 9 Gy per fraction.57 Modifications of treatment regimens using HDR fraction doses <8 Gy to point A with an increased number of fractions have shown equivalent control rates compared to LDR without increases in toxicity.57–72
Meta-analyses and published literature reviews comparing LDR and HDR brachytherapy series have confirmed equivalency of both modalities in terms of control rate and toxicity.73,74 An analysis by Orton et al. obtained from a survey of 56 institutions demonstrated 5 year survival data available for 6939 HDR patients and 3365 LDR patients to be approximately 82% for Stage I patients and 66% for Stage II patients. For Stage III patients the 5 year survival for HDR versus LDR was 47.2% versus 42.6%, respectively.74 Fu and Phillips reached a similar conclusion in their worldwide review.75 In 1999, Petereit et al. published a literature review which included 5619 patients treated with HDR brachytherapy and demonstrated 5 year pelvic control rates of 91%, 82%, and 71% in Stage I, II, and III patients, respectively, with similar survival and complication rates to previous meta-analyses.76 Although firm conclusions cannot be drawn from uncontrolled studies, this anecdotal evidence suggests that the results of HDR are at least equivalent to those achieved by LDR brachytherapy.
Four randomized studies have compared the use of HDR with the use of LDR brachytherapy in carcinoma of the cervix.77–80 Shigematsu et al. reported on 143 patients treated with HDR brachytherapy compared to 106 patients treated with LDR for stage IIB and III disease. Although the randomization technique was suboptimal (i.e., some patients who were randomized to LDR were actually treated with HDR because of limited LDR availability) the HDR arm achieved superior local control with no difference in survival compared to the LDR arm.77 In a study from India randomizing 482 patients, there was no difference in local control, survival, or severe complications between the two treatment modalities.78 However, there was a statistical difference in rectal complications, with a 20% rate with LDR versus only 6% with HDR. In 2002, Hareyama et al. reported their results of 132 patients with stage II and IIIB cervical carcinoma.79 The 5-year disease specific survival of LDR versus HDR for stage II was 87% versus 69%, respectively and for stage IIIB 60% versus 51%, respectively; however, the difference was not statistically significant. The most recent randomized study was from Thailand in 2005 evaluating 237 patients.80 There was no difference in overall survival, relapse-free survival, pelvic control, or statistical difference in complication rates between LDR and HDR brachytherapy.
Results of Imaged-Based Treatment Planning
As the use of 3D imaging for adaptive brachytherapy treatment planning for carcinoma of the cervix has only recently emerged (although rapidly increasing), there are a limited number of published results regarding the feasibility of planning or results in regards to tumor control. The GEC-ESTRO guidelines utilize MRI as it has been shown to be superior to CT in defining tumor extension in carcinoma of the cervix.81–83 However, MRI may not be readily available at most institutions throughout the world; furthermore, MRI requires specific nonmagnetic applicators that are more expensive. In this context, Viswanathan et al. recently published their results of a standardized approach to contouring between CT and MRI after applicator insertion.84 They confirmed that CT overestimates tumor volume width, resulting in a significant decrease in dose prescription parameters compared to MRI. However, contouring and dose parameters for organs at risk were similar between the two modalities. Although recognizing that MRI is superior to CT for gross tumor delineation, they have proposed CT based volume definitions for future evaluation.
Two recent reports have demonstrated the usefulness of MRI guided optimization during pulsed dose rate brachytherapy.85,86 Lindegaard et al. evaluated 21 consecutive patients and demonstrated that MRI optimization significantly improved the minimum high risk CTV dose (D100) and decreased the minimum dose to 2 cm3 of the sigmoid compared to two dimensional planning.85 Additionally, all dose volume histogram constraints were met in 76% of patients utilizing MRI optimization, versus only 14% with 3D planning. De Brabandere et al. evaluated 16 patients utilizing MRI optimization for pulsed-dose rate brachytherapy and also demonstrated a decrease in dose to COs while increasing dose to tumor compared to x-ray based plans.86 Specifically, a dose reduction of 7 Gy was achieved to the average D2cc in the bladder (+6 Gy) and sigmoid colon (+4 Gy). Simultaneously, an average dose increase of 3 Gy to the high-risk CTV was achieved.
Inverse planning as a means of dose optimization has been integrated into the process recently but is still in its infancy of development. Chajon et al., utilizing a customized vaginal mold for thirty patients, calculated dose distribution using inverse planning simulated annealing (IPSA) software.87 Inverse planning provided significantly lower doses to the ICRU bladder and rectal points compared to manual optimization methods. Although inverse planning was found to be a fast method, they cautioned its widespread use, because the optimization resulted in significant heterogeneity in dwell times. Kubicky and colleagues developed a novel technique to avoid the registration errors of MRI-CT fusion for treatment planning by using gadodiamide-filled plastic tubes inserted into the ring and tandem applicator during acquisition of the MRI to visualize the source pathway.16 An evaluation of 15 consecutive women using this novel technique along with IPSA resulted in a mean D90 of 86 Gy to the HR-CTV while maintaining a mean D1cc of 78 Gy to the bladder and 70 Gy to the rectum.
The Medical University of Vienna reported in 2007 their results of 142 out of 145 consecutive patients with Federation International de Gynecologie et d’ Obstetrique (FIGO) Stage IB-IVA, treated using MRI adaptive strategies.88 A total of 420 MRI examinations with 3D planning were performed. The latter 72 patients (period from 2001 to 2003) were planned utilizing the current GEC-ESTRO guidelines of tumor contouring and a mean D90 (dose to 90% of the high risk CTV) of 90 Gy was achieved. Continuous complete remission was 96% for patients with tumors sized 2 to 5 cm and 90% for patients with tumors sized greater than 5 cm. 95% of patients achieved complete remission with an overall 3 year progression free survival of 85%. Severe late side effects were 2% in this latter group of patients. To further validate these results, a prospective observational multicenter trial (the EMBRACE study) was started in Europe in 2008.
Carcinoma of the Endometrium
Adjuvant Radiation Therapy
High dose rate brachytherapy (either as a sole modality or as a boost to EBRT) is commonly used to treat the vaginal cuff after hysterectomy in patients with intermediate to high risk for vaginal recurrence. Indications may include high-grade adenocarcinoma histology, deep myometrial invasion, and extrauterine (including lymph node metastases) or cervical spread. The role of EBRT and/or vaginal cuff brachytherapy in endometrial carcinoma following hysterectomy in stage I disease remains controversial. The Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC) I study, originally published in 2000 (with a 10 year follow-up published in 2005), along with Gynecological Oncology Group (GOG)-99 (published in 2004), helped better define a population at an elevated risk of recurrence to support the use of adjuvant EBRT.89–91 PORTEC I randomized patients with superficial myometrial invasion (<50%) and FIGO grade 2 or 3 disease or deep myometrial invasion (>50%) and FIGO grade 1 or 2 disease to observation or whole pelvic EBRT following surgical resection.89–90 GOG-99 randomized Stage IB, IC, and occult II patients to the same therapy.91 Both studies demonstrated a decrease in local regional recurrence with EBRT (3% to 5% versus 12% to 14%) without showing an overall survival advantage. As the majority of recurrences have been located in the vagina, the PORTEC II trial was undertaken to evaluate whether adjuvant vaginal cuff brachytherapy alone could adequately replace pelvic EBRT in regards to recurrence risk, potentially improving patient’s quality of life. Eligible patients included those older than 60 years of age with FIGO stage IC grade 1 to 2 or stage IB grade 3 or those of any age with FIGO stage IIA grade 1 to 2 or grade 3 with <50% invasion. The results of the study were presented in abstract form at the 2008 American Society of Clinical Oncology annual meeting.92 With a median follow-up of 34 months 3-year actuarial rates of vaginal relapse were 0.9% in the vaginal brachytherapy group and 2.0% following EBRT. Pelvic relapse at 3 years was statistically significantly higher in the vaginal brachytherapy group with a rate of 3.6% versus 0.7% in the EBRT group. However, there were no significant differences in 3-year overall survival and recurrence free survival. Based on the data from PORTEC-2, the authors concluded that vaginal brachytherapy was a safe and reasonable alternative to EBRT, and affects QOL significantly less than EBRT; hence vaginal brachytherapy should replace pelvic EBRT in this setting. Although the options of EBRT alone, vaginal brachytherapy alone, or a combination of both exist for stage I disease, the ultimate choice requires clinical judgment regarding risk of locoregional recurrence, morbidity, costs of adjuvant therapy, and potential for salvage of patients with vaginal recurrences.
Technique of Vaginal Brachytherapy
If pelvic EBRT is given, the dose is usually about 40 to 45 Gy in 20 to 25 treatments, respectively. Brachytherapy is usually performed by a single-line source placed centrally in a vaginal cylinder. A gold fiducial marker is placed at the beginning of the procedure to indicate the position of the vaginal vault on radiographs. The largest diameter of a cylinder that comfortably first the vagina is used. Vaginal cylinder diameters of less than 3 cm are to be avoided, if possible, because of the decreased depth dose obtained with a small source to vaginal surface distance. The superior portion of the vagina (3 to 5 cm) is usually treated in most cases, although a longer length (about 8 cm or entire length of vagina) is preferable in clear cell or serous histologies.93–106
A single iridium-192 linear source, as used in a vaginal cylinder, has the potential for dose inhomogeneity at the vaginal apex due to source anisotropy. The actual clinical significance of dose anisotropy is not well defined. Some centers prefer to use an angled source, an ovoid, circular ring, or multichannel applicator to circumvent this potential problem.103
Brachytherapy Dose
The dose prescription point is either defined at the vaginal surface or at 0.5 cm from the surface of the vaginal cylinder. In either case, both doses (vaginal surface and 0.5 cm depth) are to be recorded as recommended by the ABS.103 It is not recommended to use 1.0 cm vaginal depth as a prescription dose point. The dose per fraction has varied from 4.5 to 16 Gy, and the number of fractions has varied from 2 to 7.93–102,104–108 The interval between fractions is usually 1 to 2 weeks, but more commonly once weekly. When a lower dose of about 5 Gy per fraction is chosen, a larger number of fractions is used (3 or 4) or EBRT is added, or both. The consensus report also advises the use of dose optimization for the cylinder or an angle source delivery system to be introduced to ensure uniform dose at depth and eliminate the potential dose reduction at the vaginal apex due to source anisotropy.103 The dose distribution should be optimized to deliver the prescribed dose either at the vaginal surface or at 0.5 cm depth, depending on the institutional policy. It is important to place dose optimization points not only along the lateral aspect of the vaginal wall but also at specified points about the dome of the vaginal cylinder to avoid higher vaginal apex doses which can lead to vaginal vault necrosis.2
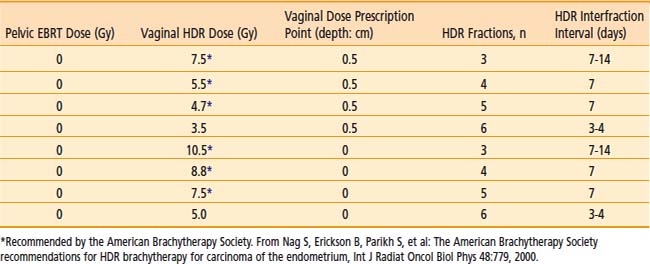
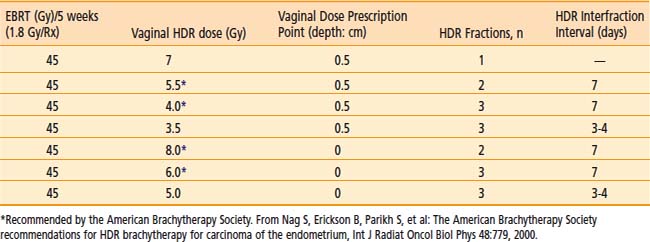
Commonly used regimen combining EBRT and HDR brachytherapy include the following: 45 Gy in 25 fractions of EBRT (four-field box technique); one, two, or three fractions of HDR spaced one week apart delivering 7 Gy, 5.5 Gy, or 4.0 Gy each, respectively, prescribed at 0.5 cm depth administered at the completion of EBRT. This allows for completion of the treatment program combining EBRT and vaginal brachytherapy within 7 weeks. A tabular summary of the treatment parameters combining EBRT and vaginal brachytherapy in the adjuvant setting is given in Table 14-7. For adjuvant vaginal HDR brachytherapy alone, a dose of 21 Gy (at 0.5 cm depth) given in three treatments spaced 1 to 2 weeks apart is commonly used and was the recommended dose in the previously mentioned PORTEC-2 trial. The ABS has made recommendations for both prescription points (0.5 cm depth, vaginal surface) (Table 14-8).
Table 14-7 Endometrial Carcinoma: Postoperative Combined External Radiation Therapy (EBRT) and Vaginal High Dose Rate Brachytherapy Regimens (American Brachytherapy Society)
Results and Morbidity
The 5-year survival results vary from 72% to 97%, depending on the usual prognostic parameters such as stage, grade, and depth of myometrial invasion.93–97,99–102,104–111 The severe (Grade III or IV) late complication rate is usually <2% and depends on the dose per fraction.93–101,104,106,107,112 Generally, doses per fraction of more than 7 Gy should be avoided (especially if EBRT is added). The incidence of vaginal shortening is also very much dose dependent, reportedly as high as 70% when 9 Gy per fraction was prescribed at 1 cm depth, to 31% when the dose was reduced to 4.5 Gy per fraction.98 The lower doses per fraction are usually given using either a larger number of fractions or in combination with EBRT to the pelvis. Sorbe et al. evaluated two different fractionation schemes (2.5 Gy × 6 versus 5 Gy × 6) in a randomized trial and found no difference in locoregional recurrence rates, but an increase in vaginal shortening, mucosal atrophy, and bleeding in the 5 Gy per fraction arm.113 Other factors that increase morbidity include the use of a small (2 cm) diameter vaginal cylinder, the addition of pelvic EBRT, and a dose specification point beyond 0.5 cm.93 Pearcey and Petereit published a literature review analyzing 1800 cases of postoperative HDR brachytherapy alone in patients with low to intermediate risk endometrial cancer.114 They found an overall vaginal control rate of 99.3%. Late morbidity was significantly higher with isoeffective doses for late responding tissues exceeding 100 Gy.
Inoperable Endometrial Carcinoma
Indications
Some patients with adenocarcinoma of the endometrium are not candidates for surgery because of severe comorbid medical problems (obesity, cardiovascular disease, diabetes mellitus, and hypertension). This subset is treated by radiation therapy. A combination of EBRT and brachytherapy or brachytherapy alone is used depending on the size of the uterine cavity, histologic grade, and other risk factors for lymph node involvement. The previously mentioned conditions that preclude these patients from surgery also place them at added risk for anesthesia and prolonged bed rest. Hence it is preferable to avoid LDR brachytherapy in this circumstance since the incidence of thromboembolic events and cardiac decompression may be significant when LDR brachytherapy is used.41
Technique
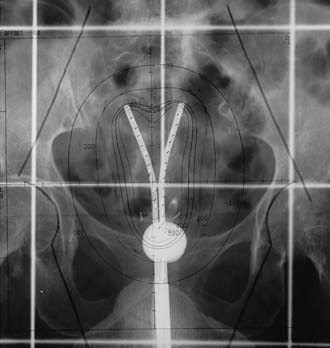
Various applicators can be used for treatment of primary endometrial cancer. A tandem and colpostat is often used; however, this applicator will not irradiate the uterine fundus homogeneously. Others have used a curved tandem, turning it to the left and right in alternate insertions. A Y-shaped applicator, comprising two abutting tandems, irradiates the fundus more evenly by allowing the tip of each tandem to lodge in each cornu (Fig. 14-9). Other possibilities include modified Heyman capsules or multiple tandems.109 The dose is commonly specified at 2.0 cm caudally from the fundus (at the midline) and 2 cm laterally, although CT or MRI based treatment planning to ensure a more homogeneous dose to the entire myometrium is preferred.
Dose Schedules
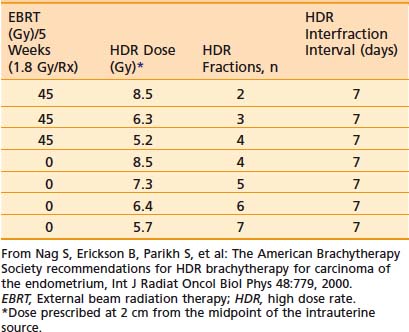
The dose per fraction has ranged from 5 to 12 Gy, and three to six fractions are commonly employed.94–95,109,115–126 The dose and/or the dose per fraction are reduced if EBRT can be added. The ABS suggested doses for HDR brachytherapy alone or in combination with 45 Gy EBRT are given in Table 14-9.103
Results
The survival at 5 years for stage I is about 70% to 80%, which is slightly lower than that obtained by surgery. A recent report by Coon and colleagues with a median follow-up of 33 months reported a 5-year cause-specific survival of 87% for patients treated with HDR twice daily with 35 Gy in five fractions without EBRT or with 20 Gy in five fractions with EBRT.127 The toxicity is higher (about 7%) when patients are treated with high doses per fraction.118,125–126
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree