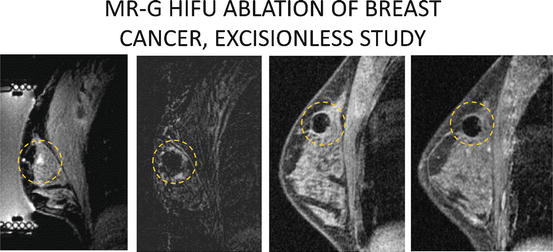
Fig. 13.1
Depiction of path of ultrasound waves from transducer to focal point with heating of target breast tissue in sonication zone
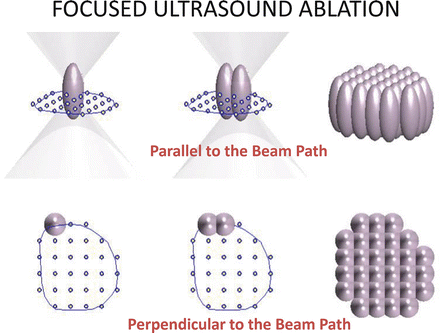
Fig. 13.2
Depiction of “cigar” shaped sonication zones arrayed side by side covering an entire tumor (blue circle) and small zone of surrounding tissue
The tissue in the FUSA treatment zone is subjected to two lethal forces: thermal energy, and mechanical stress. Over a few seconds, focused ultrasound ablation can raise the temperature of the cigar-shaped target volume to over 80 °C [2]. The rate of change, as well as the final target temperature, can be closely controlled. Maintenance of tissue temperature over 56 °C for more than one second is generally accepted to result in cell death. In addition to thermal energy, the rapidly cycling waves of rarefaction and compression at the target zone create micro-bubbles. The bubbles form and collapse many times a second, resulting in intracellular mechanical disruption. The end-result of these two forces is a precisely controlled zone of coagulative necrosis [3].
Focused ultrasound ablation can be guided via MRI or diagnostic ultrasound imaging. As mentioned above, MRI guidance allows for temperature monitoring of the ablation zone in real time using gradient-echo MRI techniques. The measurement of ablation zone temperature during ultrasound guided FUSA requires thermistor probe placement through the skin into the breast. In most studies of ultrasound guided FUSA, ablation zone temperatures were not measured [4, 5].
Description of the Procedure
Appropriate patient selection is essential. Successful MRI guided FUSA (MRgFUSA) requires that the tumor be visible on MRI and clearly delineated from the surrounding tissue. The location of the tumor must be at least 1 cm from the skin, and not adjacent to the chest wall.
The patient is positioned prone on a specially designed MRI table in which the treatment device is embedded (Fig. 13.3). The breast is positioned in the center of the MRgFUSA surface coil and an emergency stop button is placed in the patient’s hand. The breast and surface coil are lowered into a tub that contains the ultrasound transducer. The tub is filled with degassed water to ensure acoustic coupling to the transducer. During the procedure, the degassed water is maintained at 20 °C and circulated around the breast to provide active cooling of the skin. Conscious sedation is typically maintained throughout the procedure through the use of an intravenous anxiolytic/analgesic cocktail.


Fig. 13.3
The MRgFUSA device embedded in the MRI table (ExAblate 2000, InSightec, Ltd, Haifa Israel)
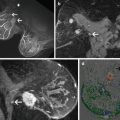
Contrast enhanced MRI images are then obtained of the breast. The tumor is identified and the treatment volume is determined (Fig. 13.4). The treatment is then delivered as a series of interlaced elliptical sonication zones delivered within the prescribed treatment area comprised of the tumor and a rim of surrounding normal tissue. Each prescribed sonication zone is individually treated and monitored, raising the temperature within it to between 50 and 90 °C. The total treatment duration is a function of the number of individual sonification zones required to treat the volume in the prescribed treatment region. Each sonification cycle requires 18–74 s, with typical total treatment time ranging between 35 and 150 min [6, 7].
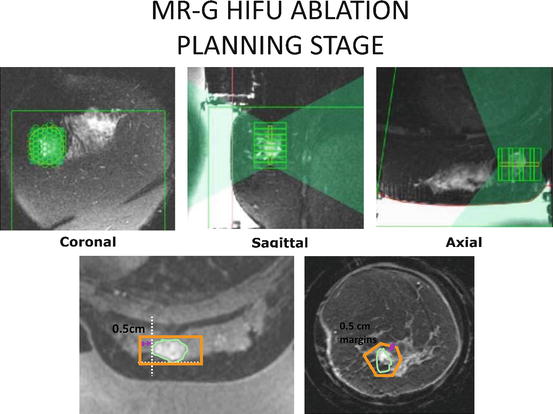

Fig. 13.4
MRgFUSA breast treatment plan (ExAblate 2000, InSightec, Ltd, Haifa Israel). The treatment is provided as a series of interlaced elliptical sonication zones delivered within the prescribed treatment area comprising of the tumor and rim of surrounding tissue. Photos courtesy of Dr. Furusawa at Breastopia, Namba Hospital, Miyazaki, Japan
Ultrasound Guided FUSA
Again, appropriate patient selection is essential. Ultrasound guided FUSA (USgFUSA) requires that the tumor be clearly visible on ultrasound. The location of the tumor must be at least 0.5 cm from the skin, or chest wall. Most studies of USgFUSA required that the tumor be more than 2 cm from the nipple [4, 5].
The patient is positioned prone on a table in which the treatment device is embedded. The breast is positioned in a tub filled with degassed water to ensure acoustic coupling to the transducer/imaging device. Conscious sedation or general anesthesia is typically maintained.
The transducer/imaging device consists of a 3.5–5.0 MHz ultrasound imaging probe situated in the center of a 12 cm therapeutic ultrasound transducer. This configuration allows for near real-time ultrasound guidance during the procedure. The transducer/imaging device is mounted to an arm that can be moved via servo motors in six dimensions.
The transducer/imaging device is used to obtain images of the breast. The tumor is identified and the treatment volume is determined. The treatment is then delivered to the tumor and a rim of surrounding normal tissue, in a similar fashion as described above for MRgFUSA. However, sonification zone temperature is not measured during USgFUSA. Typical total treatment time ranges between 45 and 180 min [4, 5].
Studies to Date
To date, there have been seven published trials evaluating MRgFUSA for the treatment of breast cancer [4, 8–13]. All but two can best be described as small feasibility studies. Gianfelice and coworkers were the first to describe a series of patients who underwent MRgFUSA followed by resection in a study completed in 2001 [6]. Twelve patients with invasive breast cancers less than 3.5 cm in greatest dimension underwent MRgFUSA of a volume of breast tissue which included the tumor and an estimated normal margin of 0.5 cm. Within 24 days of MRgFUSA, all patients underwent routine segmental resection. All patients had a minimum distance of 1 cm between the tumor and the skin or ribs. The resected specimen was evaluated with mapping of the tumor and treatment zone using three-dimensional macroscopic and microscopic histopathologic measurements combined with standard hematoxylin–eosin staining in 5 μm sections. The results from the first three patients treated in this study were not ideal, with a mean of 43.3 % of the tumor necrosis. Subsequent improvements in the targeting system used on the final nine patients in this series resulted in 88.3 % mean tumor volume necrosis. Two of the final nine patients had no residual viable tumor. Two patients in this series suffered from second-degree skin burns, both under 2.3 cm in size. Four patients reported slight discomfort, and eight reported moderate discomfort on a three point scale (slight, moderate, intolerable). Based on their initial feasibility study, Gianfelice and coworkers concluded that MRgFUSA held significant promise in terms of patient tolerability, but that the observation of residual tumor at the margins of the treatment zone suggested that refinements in tumor imaging and targeting were required.
Furusawa and colleagues reported on a study of 30 women with invasive breast cancer less than 3.5 cm in greatest diameter treated by MRgFUSA [11]. The tumor and “at least a 5-mm safety margin of normal tissue” were treated, followed 5–23 days later by routine breast conserving surgery or mastectomy. All tumors were greater than 1 cm from the skin or ribs. Histopathologic analysis of the specimens was conducted in a similar fashion to the previously mentioned study by Gianfelice and coworkers [6]. There were five protocol violations resulting in 25 evaluable patients. Mean tumor necrosis was 98 % by volume (range 90-100 %). One hundred percent necrosis was observed in 15 patients (60 %), and only one patient had less than 95 % necrosis of her tumor. One patient suffered a skin burn which was excised at the time of surgery. Two patients reported mild to moderate breast pain during sonication. Furusawa and coworkers concluded that MRgFUSA was well tolerated and effective, and that in well-selected patients, it had the potential to replace lumpectomy [11].
The use of MRgFUSA as the primary treatment of breast cancer without excision was first reported by Gianfelice et al, when he described a series of 24 patients with estrogen receptor positive, non-metastatic breast cancers. Mean tumor size was 1.5 cm (range 0.6–2.5 cm) [7]. All patients in the study had refused surgery or were considered to be “at too high a risk to undergo surgery.” After consent was obtained, all patients underwent MRgFUSA followed by or concurrent with tamoxifen therapy. Patients did not undergo tumor excision. Following MRgFUSA, breast MRI’s were obtained at 10 days, 1, 3, and 6 months. Following the MRI at 6 months, image-guided core biopsies (4–8 cores) were obtained from the area of the previously treated cancer. In the event that residual tumor was identified on biopsy, a second session of MRgFUSA was provided. Fourteen of 24 patients (58 %) had no residual disease identified on core biopsy 6 months following MRgFUSA. Ten patients with positive post-ablation biopsies were retreated with MRgFUSA, and five of the 10 were core biopsy negative 1 month later. The final success rate, defined as core biopsy negative, 7 months after initial MRgFUSA treatment was 19 of 24 patients (79 %). Mean follow-up was 20.2 months (range 12–39 months). One complication, in the form of a second-degree skin burn resolving with local wound care, was reported (4 %). The treatment was well tolerated, with ten patients reporting mild pain and 14 reporting moderate pain. Pain control was thought to be satisfactory with conscious intravenous sedation. The authors of the study concluded that MRgFUSA combined with adjuvant systemic treatment was well tolerated, had low-morbidity, and was an effective treatment for small breast cancers in selected patients.
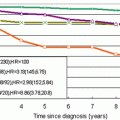
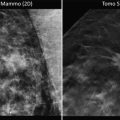
In 2010, Dr. Furusawa reported on the most recent experience in Japan with MRgFUSA for the treatment of patients with breast cancer at the Second International Symposium on MR-guided Focused Ultrasound [14]. At that time, Furusawa and colleagues had enrolled 47 patients in a prospective single arm trial of MRgFUSA followed by routine whole breast radiation therapy with no excision. All patients had a single well-demarcated tumor equal to or less than 1.5 cm in greatest dimension, well visualized on MRI, with a skin-to-tumor distance of 1.0 cm or greater. Prior to treatment, patients underwent definitive diagnosis by core biopsy and were found to be node negative by sentinel node biopsy. Patients underwent ultrasound guided core needle biopsy of the tumor site 3 weeks after completion of MRgFUSA. If no viable tumor was identified, patients received routine whole breast radiation therapy and were followed with mammography and breast MRI every 6 months (Fig. 13.5). As of October 2010, 47 patients with mean tumor size of 1.1 cm had been treated. The mean treatment duration was 108 min (range 65–209 min). Mean follow-up was 43 months, with no local recurrences or significant adverse events reported.