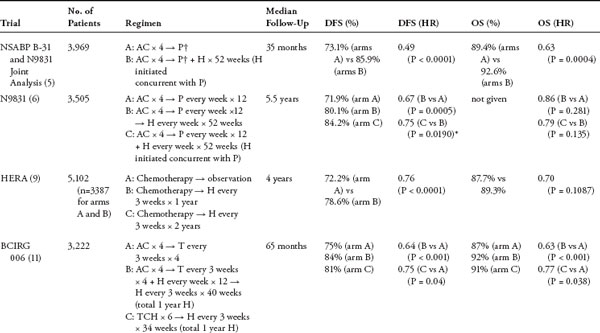
HER2-Targeted Treatment in the Adjuvant Setting for Breast Cancer Overexpression of the human epidermal growth factor receptor 2 (HER2) occurs in 25% to 30% of breast cancers and is associated with aggressive tumor behavior and poor prognosis. Trastuzumab, a monoclonal antibody against HER2, significantly improves outcome when given in combination with chemotherapy in the metastatic setting. Based on these findings, several studies have been conducted to evaluate the role of trastuzumab in the adjuvant treatment of HER2-overexpressing breast cancer. These trials demonstrate a significant improvement in disease-free survival and overall survival with the addition of trastuzumab. This chapter reviews the results of six trials incorporating trastuzumab in the adjuvant setting and highlights some of the issues regarding the optimal duration, administration (concurrently or sequentially with chemotherapy), and timing of trastuzumab initiation. The cardiac safety of trastuzumab is also discussed. Finally, ongoing studies evaluating the role of novel targeted agents in the adjuvant treatment of HER2-positive breast cancer are described. Keywords: adjuvant, epidermal growth factor receptor, trastuzumab. Approximately 25% to 30% of breast cancers over-express the human epidermal growth factor receptor 2 (HER2) (1). This overexpression is associated with more aggressive tumor biology, altered responsiveness to therapy, and poor clinical outcome including shortened survival (2). The development of trastuzumab, a humanized monoclonal antibody against HER2, is a major advance in the treatment of HER2-positive breast cancer. Trastuzumab was approved in the first-line metastatic setting after it demonstrated improved survival in combination with chemotherapy for patients with HER2-positive metastatic breast cancer (3). The pivotal clinical trial that led to the US Food and Drug Administration approval of trastuzumab in 1998 enrolled 469 patients with previously untreated HER2-positive metastatic breast cancer who were randomized to receive chemo-therapy alone (consisting of an anthracycline plus cyclophosphamide in anthracycline-naïve patients, or paclitaxel in patients previously treated with an anthracycline) or chemotherapy plus trastuzumab (3). The combination of chemotherapy and trastuzumab resulted in an improved response rate (50)% vs 32%; P .001), time to progression (median 7.4 vs 4.6 months; P .001), and overall survival (OS) (median 25.1 vs 20.3 months; P = .046) compared with chemotherapy alone. An unanticipated serious adverse event that emerged from the study was cardiac dysfunction. The rates of cardiac toxicity in patients randomized to doxorubicin and cyclophosphamide (AC) with trastuzumab versus AC alone were 27% and 8%, respectively. The rates of cardiac dysfunction in patients who received paclitaxel and trastuzumab versus paclitaxel alone were 13% and 1%, respectively. Because the combination of trastuzumab and an anthracycline resulted in unacceptably high rates of cardiac toxicity, these agents are generally not administered concurrently unless on a clinical trial. Based on impressive effcacy in the meta-static setting, four large international and two smaller European trials were conducted to evaluate the role of trastuzumab in the adjuvant setting (Table 1). Taken together, data from these trials, which include more than 14,000 patients, have since demonstrated a significant improvement in disease-free survival (DFS) and OS when trastuzumab is incorporated in the adjuvant treatment of early-stage HER2-positive breast cancer. North American Trials: NSABP B-31 and NCCTG N9831 Both the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 and North Central Cancer Treatment Group (NCCTG) N9831 trials evaluated the effcacy of adding 12 months of trastuzumab to a chemotherapy backbone consisting of AC followed by paclitaxel. The NCCTG N9831 trial randomized 3,505 patients to receive one of three regimens: AC for four cycles followed by weekly paclitaxel (P) for 12 weeks, AC for four cycles → weekly P for 12 weeks → by 52 weeks of trastuzumab (H), or AC for four cycles → weekly P for 12 weeks with 52 weeks of trastuzumab started concurrently with paclitaxel. NSABP B-31 randomized 2,030 patients to receive AC for four cycles followed by paclitaxel given every 3 weeks for four cycles, or the same chemotherapy with 52 weeks of trastuzumab started concurrently with paclitaxel. Because of similarities in the designs of both studies, results were combined in a joint analysis (4, 5). Initially, both trials required patients to have node-positive disease; the NCCTG N9831 trial was later amended to include patients with high-risk node-negative disease (defined as a tumor > 2 cm and positive for estrogen receptor [ER] or progesterone receptor [PR] or a tumor > 1 cm and negative for both ER and PR). The first interim analysis was performed at a median follow-up of 2 years. At 4 years, there was a significant improvement in DFS from 67.1% to 85.3% (hazard ratio [HR] = 0.48; 95% confidence interval [CI] = 0.39–0.59; P .0001), OS from 86.6% to 91.4% (HR = 0.67; 95% CI = 0.48–0.93; P = .015), and time to distant recurrence from 73.7% to 89.7% (HR = 0.47; 95% CI = 0.37–0.61; P .0001) in favor of the trastuzumab group (4). At 35 months of follow-up, despite a 21% crossover rate, the group treated with trastuzumab continued to show an improvement in DFS from 73.1% to 85.9% (HR = 0.49; 95% CI = 0.41–0.58; P .0001) and OS from 89.4% to 92.6% (HR = 0.63; 95% CI = 0.49–0.81; P = .0004) compared with the group that did not receive trastuzumab (5). The benefit was independent of age, tumor size, lymph node status, hormone receptor status, or tumor grade. The most recent update of N9831 was presented after a median follow-up of 5.5 years (6). DFS was 71.9% with AC → P and 80.1% with AC → P → H, corresponding to a significant 33% reduction in the risk of an event. In addition, a comparison between the two trastuzumab-containing arms in this trial was also presented, demonstrating an improvement in DFS from 79.8% in the sequential AC → P → H arm to 84.2% in the AC → PH → H arm. Although not statistically significant, this trend for a 23% reduction in the risk for a DFS event argues in favor of administering trastuzumab concurrently rather than sequentially with paclitaxel. HERA Trial The International Herceptin Adjuvant (HERA) study randomized 5,102 patients with early-stage node-positive or node-negative (if tumor > 1 cm) HER2-positive tumors to observation, trastuzumab for 1 year, or trastuzumab for 2 years following the completion of surgery and neoadjuvant/ adjuvant chemotherapy, with or without radiation (7, 8). The initial analysis presented after 12 months of follow-up for the group assigned to 1 year of trastuzumab demonstrated a significant reduction in the risk of recurrence compared with the control group with DFS of 86% versus 77% (HR = 0.54; 95% CI = 0.43–0.67; P .0001). Based on these results, 51% of patients initially assigned to observation subsequently crossed over to receive trastuzumab. After 23.5 months of follow-up, DFS was 74.3 versus 80.6% (HR = 0.64; 95% CI = 0.54–0.76; P .0001), and an improvement in OS from 89.7% to 92.4% (HR = 0.66; 95% CI = 0.47–0.91; P .0115) was seen in favor of the group receiving trastuzumab. The most recent update after a median of 4 years of follow-up revealed a 24% (HR = 0.76; 95% CI = 0.66–0.87; P .0001) reduction in recurrence risk and no significant improvement in OS (HR = 0.85; 95% CI = 0.70–1.04; P = .1087)(9). These results, however, may be confounded by the significant number of patients who had crossed over to trastuzumab. The results of 1 year versus 2 years of trastuzumab have not yet been reported. BCIRG 006 Trial Because of the increased risks of cardiac toxicity seen with both the anthracyclines and trastuzumab, there has been much interest in the development of non-anthracycline-containing chemotherapy regimens. Of the pivotal adjuvant trastuzumab trials, Breast Cancer International Research Group (BCIRG) 006 was the only one to include a nonanthracycline-containing arm. In this study, 3,222 patients with node-positive or high-risk node-negative (defined as tumor > 2 cm, ER and/or PR negative, histologic and/or nuclear grade 2 or 3, or age 35 years) HER2-positive breast cancer were randomized to receive AC for four cycles followed by docetaxel every 3 weeks (AC → T) for four cycles, AC for four cycles → T for four cycles plus 1 year of trastuzumab initiated concurrently with docetaxel (AC → TH), or docetaxel plus carboplatin plus trastuzumab (TCH) every 3 weeks for six cycles followed by trastuzumab for the remainder of 1 year. Trials of adjuvant trastuzumab in HER-positive breast cancer AC, doxorubicin and cyclophosphamide; BCIRG, Breast Cancer International Research Group; DFS, disease-free survival; ET, epirubicin and docetaxel; FEC,5- fluorouracil, epirubicin, and cyclophosphamide; FinHER, Finland Herceptin; H, trastuzumab; HERA, Herceptin Adjuvant ; HR, hazard ratio; NSABP, National Surgical Adjuvant Breast and Bowel Project; OS, overall survival; P, paclitaxel; PACS, Programme Adjuvant Cancer du Sein; T,docetaxel; TCH, docetaxel,carboplatin, and trastuzumab; V, vinorelbine. †Paclitaxel every 3 weeks × 4 in NSABP B-31; paclitaxel every week × 12 in N9831. *Statistical significance preset at P = 0.00116. The first interim analysis was conducted at a median follow-up of 23 months and demonstrated an improvement in DFS for both trastuzumab-containing arms compared with the non-trastuzumab-containing arm: 84% versus 73% (HR = 0.49; P ≤ .0001) for AC → TH and 80% versus 73% (HR = 0.61; P = .0002) for TCH (10). Results from the third planned analysis were recently reported (11). At a median of 65 months of follow-up, continued benefits in terms of DFS were reported for the two trastuzumab-containing arms: 84% versus 75% (HR = 0.64, P .001) for AC → TH and 81% versus 75% (HR = 0.75, P = .04) for TCH. A persistent OS benefit was also demonstrated (HR = 0.63, P .001 and HR = 0.77, P = .038, respectively). Although a small numerical advantage in DFS was seen for AC → TH over TCH (185) vs 214 events), this was not statistically significant, and any small benefit seen for the AC → TH arm may be offset by an increase in the number of long-term adverse events compared with TCH. There were 21 cases of congestive heart failure (CHF) in the AC → TH arm compared with four in the TCH arm. In addition, a total of nine patients in the study developed leukemia, eight of whom had received an anthracycline. The one patient who had been treated with TCH developed leukemia after receiving chemotherapy with cyclophosphamide/doxorubicin/vincristine/prednisone for lymphoma. FinHER Trial The smaller Finland Herceptin (FinHER) trial showed the benefit of a brief 9-week course of trastuzumab administered concurrently with docetaxel or vinorelbine (12). A total of 232 women with node-positive or high-risk node-negative HER2-positive breast cancer were randomized to receive three cycles of docetaxel or vinorelbine given with or without trastuzumab, followed in each group by 5-fluorouracil/epirubicin/cyclophosphamide (FEC) alone for three cycles. After adjustment for the greater number of patients with positive nodes in the trastuzumab arm, treatment with trastuzumab was associated with an improved distant DFS (80).9% vs 73.3%) compared with chemotherapy alone (HR = 0.57; P 
 ABSTRACT
ABSTRACT
 TRIALS OF TRASTUZUMAB AS ADJUVANT THERAPY
TRIALS OF TRASTUZUMAB AS ADJUVANT THERAPY

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 INTRODUCTION
INTRODUCTION