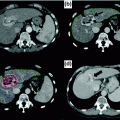
Fig. 13.1
Geographic variation in liver cancer incidence (age-standardized). [Reproduced with permission from Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 29 April 2016.]
HCC incidence in several high- and intermediate-incidence areas appears to be stabilizing or falling [1]. In China and Taiwan, this decrease is related to implementation of HBV vaccination programs and higher rates of HBV treatment. In Japan and Southern Europe, the decrease may relate to an aging cohort of HCV-infected patients [3], as the peak incidence of HCV in these countries preceded that of the USA by 10–20 years [4]. In contrast, the number of HCC cases in low-incidence areas, such as the USA, is rising [5]. Over the ten-year period from 1995 to 2004, HCC had the largest increase in incidence among solid tumors in the USA [6]. The rising incidence of HCC is largely related to the high prevalence of advanced HCV infection and non-alcoholic steatohepatitis (NASH) [1, 7]. Given the 25–30 year lag between acquisition of HCV and the development of cirrhosis, many HCV-infected patients in the USA are now presenting with complications of cirrhosis; HCV-related HCC is anticipated to continue increasing over the next 20 years [4]. Similarly, the prevalence of NASH has increased in parallel with increasing rates of diabetes and obesity; therefore, NASH-related complications, including HCC, are anticipated to increase over the next several decades.
HCC continues to have a poor prognosis, with an incidence-to-mortality ratio that approaches 1, accounting for one of the fastest growing death rates among solid tumors. A large population-based study from the USA with >15,000 patients reported 3- and 5-year survival rates were 11 and 8%, respectively, in 1992–1993, compared to 18 and 13% in 1997–1999 [6]. Similarly, 5-year survival rates in Europe were 0.9–4.9% in the early 1980s, compared to 4.6–7.9% in the mid-1990s [8]. Whereas the prognosis for most solid cancers improved from 1994 to 2003, the mortality rate for HCC nearly doubled [1, 9]. The continued poor prognosis for HCC is largely driven by high rates of late-stage presentation, when curative options no longer exist [10–15].
13.2 Risk Factors
13.2.1 Hepatitis B
HBV infection is the most common risk factor for HCC worldwide. HCC risk among HBV-infected patients is related to the mode of HBV acquisition [16, 17]. People who live in HBV-endemic areas, such as Southeast Asia or Africa, typically acquire HBV infection at birth (vertical transmission), and over 90% of these people develop chronic HBV infection. HBV carriers without cirrhosis have an annual HCC incidence of approximately 0.5%, which increases to 1% in elderly patients [18, 19]. HCC surveillance is recommended in Asian males over age 40 and Asian women over age 50 even in the absence of cirrhosis [20]. Patients from Africa are at particularly high risk, potentially related to a synergistic effect of aflatoxin exposure, and surveillance is recommended at an earlier age [21]. HBV-infected patients who are exposed to aflatoxin have a relative risk of 59.4 (95% CI 16.6–212.0) for HCC compared to those with neither exposure [22]. In contrast, most people in the USA and Europe acquire HBV infection via intravenous drug use or sexual transmission (horizontal transmission) and most experience spontaneous resolution after an acute infection. Patients with chronic HBV via horizontal transmission are at low risk for HCC in the absence of cirrhosis. In fact, >90% of HBV-infected patients who develop HCC in the USA have underlying cirrhosis [23]. Risk factors, including older age, co-infection with HCV, family history of HCC, HBV genotype, and high viral replication (high DNA levels and HBV eAg positivity) may identify subgroups who are at higher risk [24–28]. However, risk models with these variables have not been externally validated and are not ready for routine use in clinical practice [25, 26].
13.2.2 Hepatitis C
HCC can be attributed to HCV infection in approximately 60% of patients in the USA, Europe and Japan [1, 9]. HCC risk is increased 17-fold in HCV-infected patients compared to HCV-negative patients (OR 17.2, 95% CI 13.9–21.6) [29]; however, HCC risk is primarily limited to those with cirrhosis, with an annual incidence rate of 2–8% [30–32] and patients without cirrhosis are at a low risk for developing HCC [33, 34]. Several factors can moderate HCC risk in HCV-infected patients, including older age, male gender, alcohol use, and comorbid conditions such as HIV infection or diabetes [35, 36]. Although viral factors, such as genotype or viral load, do not correlate with HCC risk, successful treatment significantly reduces HCC risk among patients with HCV cirrhosis [37–39]. In patients with cirrhosis who achieve a sustained virologic response, the relative risk of HCC is only 0.27 (95% CI 0.19–0.39) [40].
13.2.3 Metabolic Syndrome and Non-alcoholic Steatohepatitis (NASH)
Several studies have linked HCC to the metabolic syndrome and its components. An analysis of SEER-Medicare demonstrated patients with metabolic syndrome have 2.1-fold increased odds (95% CI 2.0–2.3) of HCC compared to those without metabolic syndrome [41]. Similarly, a prospective study of >900,000 individuals found liver cancer mortality was 4.5-fold higher in men with BMI >35 and 1.7-fold higher in women with BMI >35 compared to normal weight individuals [42]. A meta-analysis found a pooled risk estimate of 2.4 (95% CI 1.9–2.8) among 17 case-control studies and 2.2 (95% CI 1.7–3.0) among 25 cohort studies for the association between diabetes and HCC [43]. The association between metabolic syndrome and HCC is likely driven by an increased risk of NASH as well as the direct carcinogenic potential of obesity [44]. Although it is clear NASH is a risk factor for HCC, this risk is lower than HCV-related cirrhosis. The highest HCC risk is seen among the subset of NASH patients with cirrhosis, although there are increasing reports of HCC developing in NASH patients in the absence of cirrhosis. Patients with NASH cirrhosis have cumulative HCC incidence rates of 2.4–12.8%, while NASH patients without cirrhosis have cumulative HCC mortality rates below 1% [45]. NASH cirrhosis is anticipated to be the major etiologic factor for HCC in the future as the prevalence of NASH continues increasing, in parallel with the obesity and diabetes epidemics [46].
13.2.4 Alcohol Abuse and Alcoholic Cirrhosis
Alcoholic cirrhosis is a well-recognized risk factor for HCC, and alcoholic liver disease has been reported as a contributing factor in nearly one-third of HCC cases [47–49]. However, HCC incidence rates in alcoholic cirrhosis may be overestimated given early studies predated routine HCV testing. A recent registry study from Denmark suggested HCC mortality rates may be less than 1% in alcoholic cirrhosis [50]; however, these results require external validation. Although HCC risk increases with daily alcohol intake of 40–60 grams/day [51, 52], it is unclear if lower alcohol levels increase HCC risk. An Italian case-control study with 464 HCC patients and 824 patients without liver disease found a linear increase in the odds of HCC with increasing alcohol intake, starting at 60 grams/day [53]. This study also suggested a synergistic effect between alcohol and viral hepatitis, as patients with both risk factors had a twofold increased incidence of HCC compared to those with viral hepatitis alone. Outside of promoting the development of cirrhosis, there is little evidence for a direct carcinogenic effect of alcohol [48].
13.2.5 Cirrhosis Due to Other Causes
Regardless of cause, cirrhosis is the most important risk factor for HCC [20]. The most common etiologies of cirrhosis associated with HCC include HBV infection, HCV infection, alcohol, and NASH. Hemochromatosis, primary biliary cirrhosis, autoimmune hepatitis, and alpha-1 antitrypsin deficiency are less common causes and have prevalence rates of 1–8% among patients with HCC [54–56]. Of note, patients with cirrhosis due to genetic hemochromatosis are at markedly increased risk of HCC, with a relative risk of ~20.
13.2.6 Demographic Risk Factors
In most countries, HCC rarely occurs before age 40 and the highest age-specific rates are seen in those older than 70 years [24]. Male gender is also an independent risk factor for HCC, with 2–4 times higher rates in men than women [24]. The higher incidence rates in men may be related to differential exposure to risk factors, including viral hepatitis, alcohol, and obesity [1, 57]; however, available data do not fully explain observed differences in HCC rates and a potential role for sex hormones has been suggested [58]. There are also racial/ethnic differences in the distribution of HCC. Age-adjusted incidence rates are highest in Asians (10.8 per 100,000 person-years), followed by Hispanics (7.0 per 100,000 person-years), Blacks (6.3 per 100,000 person-years), and finally, non-Hispanic Whites (2.4 per 100,000 person-years) [59]. The largest increase in HCC incidence is noted among Hispanics, whereas the smallest increase is noted among Asians.
13.3 Pathogenesis
In the last several years, there have been important advances in our understanding of HCC pathogenesis and the critical oncogenic and tumor suppressor pathways involved. The dominant paradigm suggests carcinogenesis occurs through a multistep process resulting in the progression of normal cells through pre-neoplastic states into invasive cancers [60]. The key phenotypic characteristics of cancer cells are self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis. Although the acquisition of each characteristic is thought to be necessary for the development of a full neoplastic phenotype [61], the predominant required event is unconstrained cell proliferation. This “cancer platform” concept suggests the key events driving carcinogenesis include the simultaneous development of deregulated proliferation and reduced cell death. The subsequent development of invasion, angiogenesis, metastasis, and immune evasion are secondary to the development of unrestricted proliferation [62].
13.3.1 Receptor Tyrosine Kinase Pathways
The Ras mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)-Akt kinase signaling pathways are activated by ligand binding and phosphorylation of several growth factor tyrosine kinase receptors, including the EGF receptors, the fibroblast growth factor (FGF) receptors, the hepatocyte growth factor (HGF) receptor c-met, the stem cell growth factor receptor c-kit, the PDGF receptor, and the vascular endothelial growth factor (VEGF) receptor [63]. The downstream consequences of activation of these receptors are multiple and include activation of the Grb2/Shc/SOS adapter molecule complex and downstream activation of the Ras/Raf/Erk 1/2 MAPK pathway, which results in activation of the AP-1 transcriptional activators c-fos and c-jun and consequent induction of transcription of genes that drive cell proliferation. Sorafenib is an example of an agent that blocks this pathway [64].
13.3.2 Wnt/β-Catenin Pathway
Wnts are secreted cysteine-rich glycoprotein ligands that act as ligands for the Frizzled family of cell surface receptors and activate receptor-mediated signaling pathways. The best-studied Wnt pathway activates β-catenin [65]. Activation of the Wnt pathway occurs in approximately 30–40% of HCC as a result of mutations in the β-catenin gene (12–26% of human HCC) and mutations in AXIN1 or AXIN2 (8–13% of human HCC) [66]. Wnts are involved in regulation of liver regeneration and in the maintenance and self-renewal of pluripotent stem cells and progenitor cells. Thus, they may play a role in the maintenance of the cancer stem cell compartment and are attractive targets for cancer therapy [67].
13.3.3 PI3Kinase/AKT/mTOR Pathway
Multiple cellular growth factors, including insulin, insulin-like growth factors, and cytokines such as interleukin-2, activate the PI3K family of enzymes, which produce the lipid second messenger phosphoinositol triphosphate (PIP3) and related second messengers. PIP3 in turn activates Akt/protein kinase B (PKB). Activated Akt phosphorylates several cellular target proteins, including the proapoptotic protein BAD and the mammalian target of rapamycin (mTOR) subfamily of proteins [68]. mTOR proteins in turn regulate the phosphorylation of p70 S6 kinase, a serine-threonine kinase, and the translational repressor protein PHAS-1/4E-BP. These factors coordinate translation of cell cycle regulatory proteins and promote cell cycle progression [69]. In one study, overexpression of phospho-mTOR was found in 15% of HCC tumors. mTOR phosphorylation was associated with increased expression of total p70 S6 kinase, which was found in 45% of HCC. In vitro experiments showed that rapamycin reduced p70 S6K phosphorylation and markedly inhibited proliferation of both HepG2 and Hep3B HCC cell lines. Rapamycin and other mTOR kinase inhibitors show significant activity against cancers with activated PI3K/Akt pathways [70] and are currently under investigation.
13.3.4 Angiogenic Pathways
Substances produced by cancer cells in response to local hypoxia or the interaction of the proliferating mass of cells with surrounding stromal tissue stimulate the growth of new blood vessels from the surrounding parenchyma into the tumor. Signaling pathways critical to the angiogenic process includes growth factor-mediated pathways such as VEGF and FGF receptor signaling as well as the nitric oxide signaling pathway. Hypoxia induces expression of hypoxia inducible factor 1 (HIF1) and insulin-like growth factor 2 (IGF2), both of which stimulate expression of VEGF and other growth factors [71]. HCCs are highly vascular and presumably dependent on active neoangiogenesis for their growth. In parallel with the increase in angiogenic stimuli, it has been shown that the expression of collagen XVIII, the precursor of the anti-angiogenic molecule endostatin, is decreased in larger and more vascular HCC [72].
13.3.5 Telomerase
Telomeres are specialized protein-DNA structures at the ends of chromosomes that contain long stretches of TTAGGG hexameric repeats. Telomeres prevent degradation of chromosome ends and end-to-end fusion with other chromosomes. Aging of somatic cells is associated with reduction in telomere length because of the inability of traditional DNA polymerases to replicate completely the end of the chromosomal DNA. In contrast, germ line and neoplastic cells express telomerase, an enzyme that restores telomere length. There is progressive shortening of telomeres during progression from chronic hepatitis to cirrhosis and eventually to HCC [73, 74]. Hepatocarcinogenesis is characterized by the evolution of clones of hepatocytes with increased telomerase expression and an immortalized phenotype [75, 76]. Given that it is not expressed in normal cells, telomerase-targeted therapies will likely have minimal to no significant side effects and are an attractive target for drug development.
13.3.6 Stem Cells
The acquisition of stem cell-like properties in tumors is thought to regulate cellular self-renewal potential and promote cell proliferation [77]. The Bmi-1 signaling pathway may connect this “stemness feature” to tumorigenesis. Bmi-1 belongs to the Polycomb gene group (PcG) that is involved in maintaining target genes in their transcriptional state. The ability of Bmi-1 to immortalize cells by inducing telomerase activity and promote tumorigenesis through repression of the p16INK4a and p19ARF expression indicates the involvement of the Bmi-1 “stemness” function in neoplastic proliferation [78]. Bmi-1 overexpression may cause hepatocyte immortalization through suppression of p16 and activation of human telomerase [79]; however, the exact mechanistic role of Bmi-1 in HCC tumorigenesis is not clear.
13.4 Clinical Presentation and Early Detection
The clinical presentation of HCC is driven by the degree of hepatic reserve. In patients with cirrhosis, HCC can present with hepatic decompensation including ascites, hepatic encephalopathy, or jaundice [80]. Nearly 40% of patients have HCC as their first presentation of cirrhosis [15]. In those with adequate hepatic reserve, HCC is more likely to present with tumor-related symptoms including pain, weakness, weight loss, or a palpable mass on exam [80]. Small tumors are often asymptomatic, and HCC typically becomes symptomatic when it reaches 5–8 cm in diameter [81, 82]. Outside of elevated alpha fetoprotein (AFP) levels, laboratory findings are non-specific and more related to the underlying liver disease than HCC. Extrahepatic manifestations of HCC can result from distant metastases or a paraneoplastic syndrome. Osteoclastic destruction from bone metastases can present as pain, while other sites of metastases (lung, lymph nodes, adjacent abdominal viscera) are often asymptomatic [83]. Paraneoplastic symptoms, which can occur in advanced stage tumors and serve as a poor prognostic marker, include hyperlipidemia, hypoglycemia, and hypercalcemia [84].
13.4.1 Hepatocellular Carcinoma Surveillance
Since many patients with HCC are asymptomatic at an early stage, routine surveillance in patients with known cirrhosis is important (Table 13.1). In patients with chronic HBV, HCC surveillance is supported by a randomized controlled trial among 18,816 HBV carriers who were randomized to surveillance with abdominal ultrasound and the serum biomarker, AFP, every 6 months (n = 9,373) or no surveillance (n = 9,443) [85]. Of the 86 patients who developed HCC in the surveillance group, 45% were early stage, compared to none of the 67 patients who developed HCC in the no-surveillance group (p < 0.01). HCC-related mortality was significantly lower in those undergoing surveillance (83.2 vs. 131.5 per 100,000, p < 0.01), with a hazard ratio of 0.63 (95% CI 0.41–0.98). The potential benefit of surveillance in patients with cirrhosis has only been assessed in case-control and cohort studies. Although these studies have limitations including unmeasured confounders, possible selection bias, lead-time bias, and length-time bias, they have demonstrated a consistent association between HCC surveillance and higher rates of early tumor detection, curative treatment, and improved survival [86]. Surveillance with biannual ultrasound and AFP has been demonstrated to be cost-effective in patients with compensated cirrhosis in several decision analysis models, increasing mean life expectancy with cost-effectiveness ratios between $26,000 and $55,000 per QALY [87–89].
Table 13.1
Populations in whom HCC risk is sufficiently high to warrant surveillance
Surveillance recommended |
Asian male hepatitis B carriers over age 40 |
Asian female hepatitis B carriers over age 50 |
African Blacks with hepatitis B |
Hepatitis B carriers with family history of HCC |
Cirrhosis related to hepatitis B |
Cirrhosis related to hepatitis C |
Stage 4 primary biliary cirrhosis |
Cirrhosis related to genetic hemochromatosis |
Cirrhosis related to other etiologies |
Surveillance benefits uncertain |
Hepatitis B carriers younger than 40 (males) or 50 (females) |
Hepatitis B carriers who contacted infection via horizontal transmission |
Hepatitis C carriers without cirrhosis |
Non-alcoholic fatty liver patients without cirrhosis |
13.5 Diagnosis
13.5.1 Radiologic Diagnosis
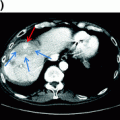
Patients with an abnormal screening test require diagnostic evaluation to determine the presence or absence of HCC (Fig. 13.2). Radiological imaging has priority in the diagnostic evaluation of patients with suspected HCC since it can facilitate HCC diagnosis, without a need for biopsy (see below), and characterizes tumor burden simultaneously. Lesions <1 cm in diameter on ultrasound are rarely HCC, so follow-up with a repeat ultrasound in 3 months is sufficient [90, 91]. For lesions ≥1 cm, triple-phase CT or dynamic contrast-enhanced MRI should be performed. If the lesion’s appearance is typical for HCC (“arterial enhancement and delayed washout”), this is sufficient for a diagnosis of HCC and no further investigation is needed (see discussion below). If the appearance is not typical for HCC, a second contrast-enhanced study or biopsy should be performed. Patients with a high suspicion for HCC but negative biopsy should be followed with serial contrast-enhanced imaging. If the lesion enlarges but remains atypical appearing, repeat biopsy can be considered. A study validating this approach found the first biopsy was positive in 70% of patients with HCC; however, up to 3 biopsies were required in some cases [92].
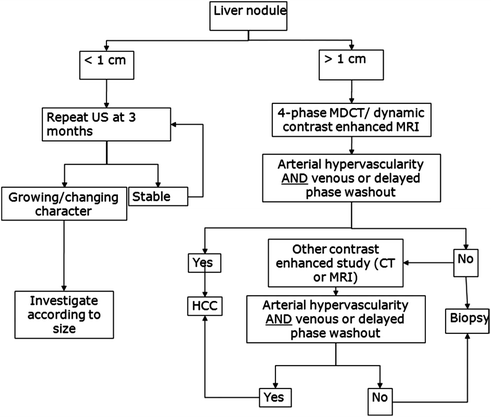

Fig. 13.2
Diagnostic algorithm (AASLD guidelines) for hepatocellular carcinoma. [Reprinted from Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. With permission from John Wiley and Sons]
As noted above, patients with a positive surveillance test should be evaluated with triple-phase CT or contrast-enhanced MRI. Established protocols for CT and MRI define the amount and method of contrast administration, timing of the studies after contrast administration, and thickness of slices required for adequate resolution. Several studies have compared performance characteristics of CT and MRI as diagnostic modalities for HCC [100–103]. The sensitivity of MRI is 61–95% compared to 51–86% for triple-phase CT [104]. The role of other imaging modalities, including contrast-enhanced ultrasound, remains debated [105, 106]. Positron emission tomography has poor performance for HCC diagnosis and is not included in the diagnostic algorithm [107].
HCC lesions enhance more than the surrounding liver in the arterial phase and less than the hepatic parenchyma in the venous and delayed phases. Arterial enhancement is an essential characteristic of HCC but is non-specific, as it can be seen in other hypervascular hepatic lesions, such as hemangioma and focal nodular hyperplasia as well as some metastases [108, 109]. Delayed washout is the strongest predictor of HCC among those with an arterial-enhancing lesion (OR 61, 95% CI 3.8–73) [93]. The presence of arterial enhancement and delayed washout had a sensitivity of 89% and specificity of 96% for HCC. The phenomenon of “arterial enhancement and delayed washout” is related to the differential blood supply of the tumor compared to the surrounding liver [102, 110]. The liver obtains ~75% of its blood supply from the portal vein and the remainder from the hepatic artery. As a dysplastic nodule transitions to HCC, there is a gradual reduction in the portal blood supply to the nodule and an increase in arterial blood flow from hepatic artery branches through neoangiogenesis [111, 112]. In the arterial phase, HCC receive contrast-containing arterial blood, while arterial blood to the surrounding liver is diluted by venous blood without contrast. In the portal venous and delayed phases, HCC tumors do not receive any contrast given lack of a portal venous blood supply, while the surrounding liver receives portal blood with contrast.
Lesions between 1 and 2 cm demonstrate typical imaging characteristics less often than larger lesions and can pose the most difficulty for diagnosis. Many of these lesions are not malignant; however, some small HCC lesions can have aggressive behavior leading to vascular invasion and poor survival if not diagnosed early [93–96]. Although requiring one characteristic contrast-enhanced study to make a diagnosis of HCC in 1–2 cm lesions has a lower positive predictive value than requiring two studies, the positive predictive value still exceeds 90% [92, 97, 98]. Serste and colleagues validated this approach in a study among 74 patients with 1–2 cm nodules, of whom 47 had HCC [99]. The sensitivity and specificity of characteristic findings on one imaging study, for the detection of HCC or high-grade dysplatic nodules, was 96 and 100%, respectively, compared to 57 and 100% if characteristics findings were required on both studies. Liver biopsy provided an accurate diagnosis in the 21 (28%) patients with discordant imaging findings on CT and MRI. Chapter 2 describes the Liver Imaging Reporting and Data System (LI-RADS), which serves as a guideline for radiographic diagnosis of liver lesions, in more detail.
Although most HCC exhibit arterial enhancement and delayed washout, some HCC have an atypical presentation. For example, hypovascular HCC enhances less than the surrounding liver in both arterial and venous phase imaging [113, 114]. This appearance is related to immature neoangiogenesis and incompletely established arterial supply. As the lesion matures, the blood supply becomes more arterialized and it will usually exhibit characteristic features [115].
13.5.2 Histologic Diagnosis
Biopsy should be considered in patients with a suspicious liver mass whose appearance is not typical for HCC on contrast-enhanced imaging. Percutaneous biopsy has a sensitivity of 67–100% and specificity of 100% for HCC diagnosis [116–118]. In a study of >2000 biopsies, the most common complication was post-procedural bleeding, but this occurred in only 0.4% of patients [116]. Biopsy of HCC was initially reported to have a 2.7% incidence of needle tract tumor seeding [119]; however, use of a coaxial needle technique significantly reduces this risk [120].
Large HCC can often be diagnosed through imaging alone; however, smaller lesions are more likely to have non-characteristic imaging and may require biopsy to make a diagnosis. In the setting of cirrhosis, there is often a stepwise progression from cirrhotic regenerative nodule to dysplastic nodule to HCC. Some dysplastic nodules have concurrent foci of HCC at time of initial presentation, and one-third of high-grade dysplastic nodules will progress to HCC over a two-year follow-up period [121]. Dysplastic nodules can be classified as low-grade or high-grade, with the risk of HCC increasing with the degree of dysplasia. Malignant transformation rates are 25% in low-grade dysplastic nodules, compared to rates as high as 63% in high-grade dysplastic nodules; however, the latter figure may be difficult to interpret given high-grade dysplastic nodules can be difficult to distinguish from well-differentiated HCC [122, 123]. Not all dysplastic nodules will progress to HCC, as 15% of nodules can disappear on follow-up.
The International Consensus Group for Hepatocellular Neoplasia developed definitions for each of these lesions, leading to increased global standardization of nomenclature among pathologists [124]. Low-grade dysplastic nodules appear distinct from the surrounding liver and can be nodular appearing due to a peripheral fibrous scar. These nodules are characterized by a mild increase in cell density without cytologic atypia or architectural changes. Unpaired arteries can sometimes be present in small numbers [125]. High-grade dysplastic nodules are more likely to demonstrate a nodular appearance, although they lack a true capsule. High-grade dysplastic nodules are characterized by the presence of cytologic atypia and architectural changes, but the atypia is insufficient for a diagnosis of HCC. They often exhibit a combination of increased cell density, irregular trabeculae, small cell change, and unpaired arteries but should not have evidence of stromal invasion [126]. Immunostaining for keratins 7 or 19 may be used in difficult cases to differentiate stromal invasion versus ductular reaction and pseudo-invasion [127]; if present, the stains would support a diagnosis of HCC.
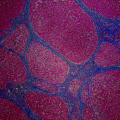
Early HCC are vaguely nodular and are characterized by a combination of histologic features including: (1) increased cell density more than two times that of the surrounding tissue, with an increased nuclear to cytoplasm ratio and irregular thin trabecular pattern, (2) varying numbers of intratumoral portal tracts, (3) pseudoglandular formation, (4) diffuse fatty change, and (5) unpaired arteries [125, 128, 129]. Features of HCC may be present diffusely throughout the lesion but may be restricted to only a portion of the nodule. Furthermore, all of these findings may be found in both early HCC and high-grade dysplastic nodules. Therefore, stromal invasion remains the most helpful feature to distinguish early HCC and high-grade dysplastic nodules. Figure 13.3 shows various histological features of HCC.
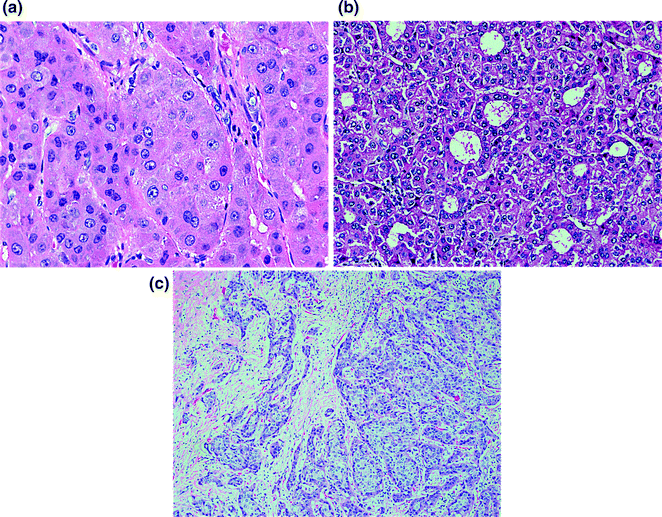

Fig. 13.3
Histologic characteristics of hepatocellular carcinoma including thickened hepatocyte cords (a), pseudoglandular formation (b), and stromal invasion (c)
Staining for several biomarkers, including glypican-3 (GPC3), heat shock protein 70 (HSP70), and glutamine synthetase (GS), has been proposed to help distinguish HCC from high-grade dysplastic nodules [130–135]. GPC3 is an oncofetal protein that is expressed in 60–90% of HCC, although at lower rates around 50% in well-differentiated HCC. HSP70, a potent anti-apoptotic protein, is expressed in up to 80% of early HCC in resection specimens but less than 50% of cases on biopsy. GS, which correlates with beta-catenin mutations, has a stepwise increase in expression from precancerous to early and advanced HCC. GS expression has been reported in 13–70% of early HCC but only 10–15% of high-grade dysplastic nodules. The diagnostic accuracy of a panel of these 3 markers was assessed among a cohort of 186 patients with regenerative nodules (n = 13), low-grade dysplastic nodules (n = 21), high-grade dysplastic nodules (n = 50), very well-differentiated HCC (n = 17), well-differentiated HCC (n = 40), and poorly differentiated HCC (n = 35) [131]. When two markers were positive, the accuracy for HCC detection was 78.4%, with 100% specificity. This panel was subsequently prospectively validated among a cohort of 60 patients who underwent biopsy for liver nodules smaller than 2 cm [136]. When at least two of the markers were positive, the sensitivity and specificity were 60 and 100%, respectively; however, the panel only corrected 1 of 3 false positives using conventional pathology analysis. Although this panel appears promising, its clinical utility over conventional pathology has yet to be established.
Recent advances in genomics could provide novel tools to further improve HCC diagnosis. Application of real-time reverse transcription polymerase chain reaction has demonstrated differential expression of genes in high-grade dysplastic nodules and early HCC. For example, a 3-gene set including GPC3, survivin, and LYVE1 had a discriminatory accuracy of 94% between dysplastic nodules and early HCC [137].
13.6 Staging
One of the central factors driving prognosis in patients with HCC is tumor burden. In most solid tumors, staging is determined at time of surgery and by pathologic examination of a resected specimen, leading to the Tumor Node Metastasis (TNM) classification [138]. However, the TNM staging system in HCC fails to account for the degree of liver dysfunction and patient performance status [139]—two important dimensions that cannot be ignored in patients with HCC. Several other staging systems have been proposed, including the Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), and Japan Integrated Staging (JIS). Although there is not one universally accepted staging system, the BCLC (Fig. 13.4) may offer the most prognostic information because it includes an assessment of tumor burden, liver function, and patient performance status [139, 140] and has been endorsed by the American Association for the Study of Liver Diseases (AASLD) [141]. The prognostic ability of the BCLC has been validated in European, American, and Asian populations [139, 140, 142]. In a study comparing the prognostic ability of seven staging systems, the BCLC was found to have the best predictive power for survival [139]. Median survival for patients with BCLC stage D tumors was only ~5 months, which was significantly shorter than the 10-month median survival for those with BCLC stage C tumors (p = 0.01). Patients with BCLC stage B tumors had a median survival of ~27 months (p = 0.04 vs. BCLC stage C tumors) and BCLC stage A patients had a median survival >4 years (p < 0.001 vs. BCLC stage B). In addition to its strong prognostic ability, the BCLC is the only staging system that has been linked to an evidence-based treatment algorithm (Fig. 13.4). However, the validity of the BCLC staging system will need to be re-evaluated in the future with progress in both risk stratifications and treatment options.
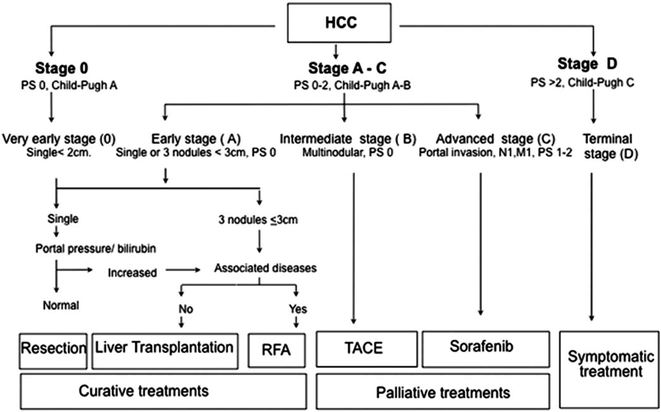

Fig. 13.4
Barcelona clinic liver cancer (BCLC) staging system for hepatocellular carcinoma [Reprinted from Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. With permission from John Wiley and Sons]
13.7 Treatment
There have been significant advances in HCC treatment over the past ten years, with improvements in technology and patient selection. Curative therapies include surgical resection, liver transplantation (LT), and locoregional ablative techniques such as radiofrequency ablation (RFA), microwave ablation (MWA), and stereotatic body radiation therapy (SBRT); each approach offers the chance of complete response and long-term survival. Palliative therapies, which typically slow tumor progression and prolong survival, include transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and systemic chemotherapy.
13.7.1 Hepatic Resection
Surgical resection is the treatment of choice for non-cirrhotic patients with HCC. Despite more advanced tumor stage at diagnosis, HCC patients without cirrhosis are more likely to be resection candidates due to lower risk of liver failure [143]. Whereas >40% of HCC in Southeast Asia occurs in the absence of cirrhosis, this accounts for less than 10% of HCC in the USA [23, 144]. Consequently, widespread use of resection is limited in Western countries.
Patients with limited hepatic reserve are at risk for hepatic decompensation, so careful patient selection is crucial. Although perioperative mortality rates after resection have improved over time, hepatic decompensation occurs in 4–5% of patients [145, 146]. It is important to consider both quality and quantity of the future liver remnant (FLR) after resection. In patients with limited fibrosis, the risk of postoperative morbidity is low if FLR exceeds 30%; however, an FLR of 40% is typically required in patients with cirrhosis [147]. In patients with insufficient FLR, portal vein embolization (PVE) can be a useful adjunct to promote hypertrophy of the unaffected hepatic lobe [148, 149]. Quality of FLR is based on an assessment of hepatic function and degree of portal hypertension. Patients with Child Pugh A disease have significantly better survival after resection than patients with Child Pugh B or C disease [147, 150]. However, Child Pugh score alone has a floor effect and is unable to identify Child Pugh A patients at risk for postoperative liver failure. Five-year survival rates are only 25% in patients with portal hypertension and bilirubin >1 mg/dL, compared to 74% in patients without portal hypertension and normal bilirubin levels [151]. Whereas some studies have used invasive means, such as hepatic vein gradient greater than 10 mmHg, to define portal hypertension, others have used platelet count <100,000/mm3 as a non-invasive surrogate marker [152–155].
The efficacy of surgical resection is also linked to tumor stage. Five-year survival rates are only 10% in patients with vascular invasion compared to 41–57% in those without vascular invasion. Similarly, patients with tumors <2 cm in diameter have 5-year survival rates of 54–93%, whereas those with 2–5 cm tumors have 38–53% 5-year survival rates, and those with tumors >5 cm have 5-year survival rates below 39% [156–159]. Although resection yields 5-year survival rates of nearly 70% (Table 13.2), it is limited by high tumor recurrence rates, as high as 50–70% after 5 years [160]. Early recurrences within 2 years are likely due to dissemination of the original tumor, whereas late recurrences after 2 years are more likely “de novo” HCC. Early recurrence risk is associated with tumor factors (pre-operative tumor stage), whereas viral factors (e.g., persistent HCV infection) and degree of liver dysfunction drive late recurrences [161, 162].
Table 13.2
Selected cohort studies of surgical resection for hepatocellular carcinoma
Reference | Number of patients | Proportion of patients with cirrhosis (%) | Proportion of patients with child pugh A status (%) | Overall survival |
|---|---|---|---|---|
Itamoto [237] | 136 | 100 | 77.2 | 70% at 5 years |
Poon [238] | 204 | 100 | 95.6 | 68% at 5 years |
Taura [239] | 293 | 56.7 | 87.4 | 61% at 5 years |
Kamiyama [240] | 321 | 39.3 | 96.6 | 74% at 5 years |
Park [241] | 213 | 100 | 100 | 69% at 5 years |
Huang [242] | 115 | 65.2 | 92.2 | 76% at 5 years |
Sakaguchi [243] | 111 | 70.3 | 83.8 | 78% at 5 years |
Zhou [244] | 1018 | 100 | 97.6 | 67% at 5 years |
A Cochrane review found 12 randomized controlled trials assessing the role of adjuvant or neoadjuvant therapy with resection [163]. Lower recurrence rates were observed across studies, although only three reported significant reductions in recurrence. Overall, there is insufficient evidence for neoadjuvant or adjuvant regimens with resection. In contrast, several studies, including 5 randomized controlled trials, have demonstrated HCV treatment after resection or ablation (i.e., secondary prevention) significantly reduces HCC recurrence rates [164]. Patients with SVR have HCC recurrence rates of approximately 35%, which is significantly lower than the 61% recurrence rate among non-responders (p = 0.005) [164].
13.7.2 Liver Transplantation (LT)
LT offers the unique ability to not only treat HCC, but also correct the underlying liver disease, thus minimizing the risk of tumor recurrence. In a landmark study, Mazzaferro and colleagues demonstrated long-term survival was possible in patients with limited tumor burden [165]. Among patients with one tumor <5 cm in diameter or 2–3 tumors each <3 cm in diameter and without portal vein invasion or extrahepatic metastases, 4-year survival rates of 85% were achieved. These criteria, known as the Milan criteria, form the basis of priority listing status for LT in the USA. When these criteria are applied in clinical practice, several studies show recurrence rates are less than 15% and 5-year survival rates approach 60–70% (Table 13.3) [166].
Table 13.3
Selected cohort studies of liver transplantation for hepatocellular carcinoma
Reference | Number of patients | Transplant criteria | Recurrence rate | Overall survival |
|---|---|---|---|---|
Mazzaferro [245] | 60 | Milan | 7% at 4 years | 75% at 4 years |
Herrero [246] | 47 | Expanded criteria | 13% at 5 years | 79% at 5 years |
Todo [247] | 316 | Expanded criteria | 13% at 3 years | 69% at 3 years |
Pelletier [248] | 2552 | Milan | Not reported | 65% at 5 years |
Strict selection criteria have been maintained given the need to obtain the maximum benefit from a limited number of available organs. However, some believe the Milan criteria may be too restrictive and have proposed expanding selection criteria to include patients with larger tumors [167–169]. For example, the University of California San Francisco (UCSF) criteria include patients with a single lesion <6.5 cm or 2–3 lesions, each <4.5 cm with a maximum tumor burden of 8.0 cm [170]. The benefit to patients beyond Milan criteria must be weighed against the harm from delaying transplantation in others on the waiting list. The harms of expanding selection criteria typically outweigh the benefits when 5-year post-transplant survival rates fall below 61% [171]. Although promising results have been reported from single-center cohort studies, patients exceeding Milan criteria have higher post-transplant mortality (HR 1.68, 95% CI 1.39–2.03) [172], with 5-year post-transplant survival rates of only 38% [173].
An alternative approach to expanding transplant criteria is downstaging larger tumors to Milan criteria using TACE, TARE, or local ablative therapy [174]. In a prospective study among 61 patients with T3 lesions, downstaging was successful in 43 (70%) patients [175]. The 4-year survival of the entire cohort was 69 and 92% in the 35 patients who underwent LT. This process theoretically selects tumors with more favorable biology that responds to downstaging treatments and would likely do well after LT [176]. Although few data compare the effectiveness of downstaging modalities, a single-center analysis with 86 patients suggested TARE outperforms TACE in terms of successful downstaging (58% vs. 31%, p = 0.02) and overall survival (35.7 vs. 18.7 months, p = 0.18) [177]. Further large prospective studies are needed to define the role and optimal technique for successful downstaging.
In regions with prolonged waiting times for LT, intrahepatic tumor growth, vascular invasion, or extrahepatic metastases may lead to dropout from the waiting list while awaiting an organ. In regions with waiting times exceeding 12 months, nearly 25% of HCC patients experience dropout [178, 179]. Accordingly, the proportion of LT recipients receiving “bridging therapy” while on the waiting list increased from 37.3% in 2003 to 58.1% in 2008 [180]. Although there is not any proven post-transplant survival advantage in treating HCC patients while awaiting LT, “bridging” therapy may reduce the risk of dropout [180, 181].
13.7.3 Local Ablative Therapies
Local ablation therapy is an alternative for patients with early HCC who are not eligible for resection or LT. RFA involves the use of electromagnetic energy deposition via a percutaneous probe to induce thermal injury to the tumor, leading to local coagulation necrosis [182]. Excellent long-term outcomes have been reported after RFA (Table 13.4) [183–185]. In a study among 1170 patients with HCC, complete tumor ablation was achieved in 99.4% after a median number of 2 RFA sessions [186]. Five- and ten-year survival rates were 60.2 and 27.3%, respectively; however, 74.8% of patients had recurrence within five years of the procedure.
Table 13.4
Selected cohort studies of local ablative therapy for hepatocellular carcinoma
Reference | Number of patients | Rate of local tumor progression | Overall survival |
|---|---|---|---|
Lencioni [249] | 206 | 10% | 41% at 5 years |
Tateishi [250] | 319 | 9% | 54% at 5 years |
Chen [251] | 256 | Not reported | 41% at 5 years |
Choi [252] | 570 | 12% | 58% at 5 years |
Livraghi [253] | 216 | 1% | 55% at 5 years |
N’Kontchou [254] | 235 | 12% | 40% at 5 years |
Three RCTs demonstrated similar 3-year survival rates after percutaneous ablation and resection in patients with early HCC, although there was a consistent trend in improved disease-free survival after resection [187–189]. The choice between the treatments depends on local expertise and the risk of local recurrence and perioperative mortality [190]. A Markov model concluded resection is the best therapeutic option, except in cases where patients were older than 70 years, resection perioperative mortality exceeded 30%, negative margins were achieved in less than 60% of patients, or RFA could be performed at least 60% of time for recurrence [191].
A major limitation of RFA is its poor efficacy in large tumors, with a lower chance of complete necrosis in tumors exceeding 3 cm. Tumors >3 cm require repositioning of the electrode or multiple treatment sessions to obtain clear ablation margins. Lesions >5 cm only have 50% chance of complete response even with a more aggressive approach [192–194]. Accordingly, RFA yields 3- and 5-year survival rates of 84 and 65% for tumors <3 cm compared to 71 and 47% in larger tumors (p < 0.001) [186]. TACE prior to RFA has been proposed to decrease HCC blood flow and heat dispersion to increase the size of RFA necrosis, although well-conducted randomized trials are still necessary [195]. Another limitation of RFA is an inability to treat some HCC due to tumor location. Subcapsular and surface HCC and those adjacent to the gallbladder are associated with higher rates of incomplete ablation and may be associated with higher complications rates [196–198]. Similarly, tumors adjacent to large vessels can have a 50% lower chance of complete response to RFA, as the vessel acts as a “heat sink” for the radiofrequency energy [199]. Finally, RFA is associated with potential adverse events including pleural effusion, peritoneal bleeding, and a 0.3% risk of procedure-related mortality [200–202].
MWA is an alternative therapy, involving ultrasound-guided placement of an electrode and microwave treatment to induce regional necrosis surrounding the HCC [203, 204]. MWA can overcome some limitations of RFA, achieving wider ablative zones, and avoiding heat sink effects [205]. A randomized trial comparing RFA and MWA among 72 patients found similar rates of complete response (96% vs. 89%, p = 0.26), residual foci of untreated disease (8.3% vs. 17.4%, p = 0.20), and complications (2.8% vs. 11.1%, p = 0.36); however, the number of required treatment sessions was significantly lower in the RFA group (1.1 vs. 2.4, p < 0.001) [206]. There have been advances in microwave ablation since this study, so repeat trials comparing RFA and MWA are needed. Other novel therapies, such as irreversible electroporation (IRE), have also shown promise as alternative techniques but further data are needed.
13.7.4 Transarterial Chemoembolization (TACE)
TACE involves selective delivery of intra-arterial chemotherapy into the tumor, followed by embolization with a goal of inducing tissue necrosis. TACE is a primary treatment for patients with preserved liver function (Child A or B) and tumors that are not amenable to surgical resection, LT, or local ablative therapies, in the absence of vascular invasion or distant metastases [207–209]. TACE carries a significant risk of hepatic ischemia in patients with hepatofugal blood flow and/or main portal vein thrombus. Although this has traditionally been considered a contraindication to TACE, subsequent reports have suggested this may be performed in select patients with preserved liver function [210, 211].
Objective response rates range between 16 and 60%, and fewer than 2% of patients achieve a complete response [207–209]. The residual tumor recovers its blood supply and continues to grow, necessitating repeated TACE treatments at regular intervals. TACE results in slower rates of tumor progression, which translates into lower rates of vascular invasion and distant metastases. A meta-analysis of randomized trials demonstrated a survival benefit for TACE in patients with intermediate stage tumors ( Table 13.5). TACE results in a significantly prolonged two-year survival of 63% compared to 27% with supportive care (p < 0.001) [209].
Table 13.5
Selected randomized trials of TACE for hepatocellular carcinomaa
Reference | Number of patients | Proportion child A | Objective response rate (TACE vs. comparison arm) | 2-year survival |
|---|---|---|---|---|
Pelletier [255] | 42 | 88% | 33% vs. 0% | Not reported |
GETCH [256] | 96 | 91% | 16% vs. 5% | 38% vs. 26% |
Bruix [257] | 80 | 82% | 55% vs. 0% | 49% vs. 50% |
Pelletier [258] | 73 | 76% | 9% vs. 2% | 24% vs. 26% |
Lo [259] | 79 | Not reported | 11% vs. 1% | 31% vs. 11% |
Llovet [260] | 75 | 70% | 14% vs. 0% | 63% vs. 27% |
Although attempts are made to be as selective as possible, there is often injury to surrounding hepatic parenchyma resulting in post-embolization syndrome with pain, nausea, and low-grade fevers [212]. The post-embolization syndrome is usually self-limited to 48–72 h, and usually resolves with pain medications and hydration. The degree of side effects and risk of hepatic toxicity may be determined by the type and frequency of the TACE regimen, with high variability in procedural technique between centers. There is also variability in the choice of chemotherapeutic agent (doxorubicin alone vs. combination with mitomycin-C or 5-fluorouracil vs. bland embolization), embolizing agent (gel foam vs. microparticles), TACE re-treatment schedule (ranging from every 2 months to 6 months), and degree of selectivity (ranging from super-selective to lobar TACE).
The introduction of drug-eluting beads (DEB-TACE), which can be more embolic and maintain higher intratumor doxorubicin levels, may help reduce some heterogeneity between centers [213]. DEB-TACE involves embolic microspheres that sequester chemotherapeutic drugs, such as doxorubicin, and release them in a controlled and sustained fashion. A RCT among 212 patients with intermediate stage HCC found DEB-TACE had similar response rates to conventional TACE (27% vs. 22% complete response, 25% vs. 21% partial response) and similar treatment-related serious adverse effects rates (24% vs. 30%) [214]. However, the DEB-TACE group had lower rates of post-embolization liver toxicity and systemic doxyrubicin effects, such as alopecia. DEB-TACE was superior to bland embolization in an RCT among 84 patients, with higher complete response rates at 6 months (27% vs. 14%), lower recurrence rates at 12 months (46% vs. 78%), and significantly longer time-to-progression (42 weeks vs. 36 weeks, p = 0.008) [215]. A study of 104 patients treated with DEB-TACE validated its safety (9.6% major complication rate) and efficacy (median survival 48.6 months) [216].
13.7.5 Systemic Therapy
Several chemotherapeutic agents have been investigated as potential therapies for patients with advanced HCC who are not candidates for local therapy [217]. Studied agents included but are not limited to doxorubicin, tamoxifen, cisplatin, seocalcitol, and nolatrexed. However, these agents failed to demonstrate notable response rates or improvement in survival over best supportive care. In 2008, sorafenib, a multikinase inhibitor, was the first and only agent to date that has been shown to significantly improve survival benefit in patients with advanced HCC [218].
There have been two large randomized trials demonstrating a survival benefit with sorafenib (Table 13.6). The SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) trial was terminated early, after finding median survival improved from 7.9 months with placebo to 10.7 months with sorafenib (HR 0.58, 95% CI 0.45–0.74) [218]. Time-to-progression was significantly prolonged from 2.8 months in the placebo group to 5.5 months among those receiving sorafenib (p < 0.001). The majority of patients included in this trial had Child A cirrhosis (95%) and good performance status (92%) with advanced tumors (53% extrahepatic spread and 70% vascular invasion). Another randomized trial with sorafenib in patients with advanced HCC was performed in Asia, in which there were significantly higher rates of patients with HBV infection [219]. Median survival was 6.5 months in the patients treated with sorafenib, compared to 4.2 months in those who received placebo (HR 0.68, 95% CI 0.50–0.93).
Table 13.6




Selected randomized trials of sorafenib for hepatocellular carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree