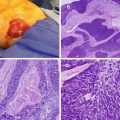
Fig. 15.1
MRI appearance of an HCC lesion in the left liver lobe demonstrating characteristic enhancement in the early arterial phase (a), with subsequent contrast washout in the delayed, portal venous phase (b)
For patients with cirrhosis, lesions greater than 1 cm in size that display these hallmark imaging characteristics are diagnostic of HCC and do not require a confirmatory tissue biopsy. For patients with liver nodules suspicious for HCC but lacking these imaging features on one imaging study, a second modality should be considered. If imaging remains inconclusive, or for patients with liver nodules arising in the absence of underlying cirrhosis, histologic confirmation by core needle biopsy is necessary for pathologic diagnosis. Improved imaging technology and adoption of the diagnostic criteria above have helped limit the need for invasive percutaneous biopsy, which carries risks of potential complications such as tumor rupture or biopsy track seeding, estimated at 0–5.1 % [11, 12]. Serum alpha-fetoprotein (AFP) may play a role as an adjunctive test in patients with suspicious liver lesions, with some degree of AFP elevation observed in the majority of patients, but elevated AFP levels are not a requisite component of the most recent iteration of diagnostic criteria [13]. An initially elevated AFP level, however, can be of benefit to gauge tumor response to therapy and monitor for future recurrence following treatment.
Cross-sectional imaging also provides information regarding morphologic features of the HCC, including tumor focality (uninodular vs. multinodular), macrovascular invasion, presence of main portal or hepatic venous thrombus, and involvement of the biliary tree, as well as potential lymph node involvement or extrahepatic spread of disease (Figs. 15.2 and 15.3). Chest imaging is also appropriate, as HCC commonly metastasizes to the lungs. Clinical management of patients with HCC requires an understanding of these tumor-specific features as well as the severity of their liver dysfunction and natural history of cirrhosis.
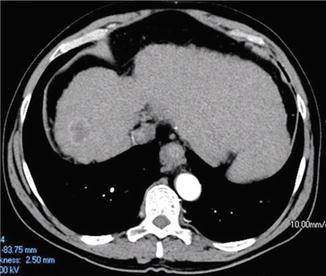
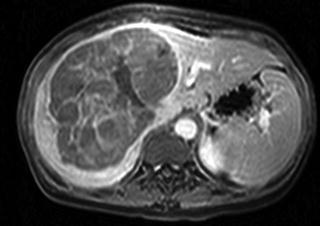

Fig. 15.2
Small HCC lesion arising in the background of a cirrhotic liver. In the absence of any evidence of vascular invasion or distant metastases, this lesion would meet Milan Criteria

Fig. 15.3
A large HCC lesion arising in the setting of otherwise normal-appearing liver parenchyma
Patients with HCC typically have some degree of underlying liver disease, the severity of which can be quantified on the basis of the Child-Turcotte-Pugh (CTP) score or the Model for End-Stage Liver Disease (MELD) score. The CTP score stratifies patients with underlying cirrhosis on a scale of 5–15 points and incorporates points assigned for quantitative serum values for bilirubin, albumin, and INR (international normalized ratio) as well as the more subjective variables of ascites and encephalopathy (Table 15.1) [14, 15]. Patients with CTP Class A cirrhosis (score of 5–6 points) have a 2-year mortality risk of 10 % versus 20–40 % for those with Class B cirrhosis (score of 7–9 points) or 50–80 % for those with Class C cirrhosis (score 10–15 points) [14].
Table 15.1
Child-Turcotte-Pugh (CTP) classification of hepatic function
Variable | 1 Point | 2 Points | 3 Points |
|---|---|---|---|
Serum bilirubin (mg/dL) | <2.0 | 2.0–3.0 | >3.0 |
Serum albumin (g/dL) | >3.5 | 2.8–3.5 | <2.8 |
INR | <1.7 | 1.7–2.3 | >2.3 |
Ascites | Absent | Slight | Moderate–severe |
Encephalopathy (grade) | None | Mild (I–II) | Severe (III–IV) |
The MELD score, calculated from the patient’s serum creatinine, bilirubin, and INR values using a linear regression model, is more objective than the CTP score as it does not incorporate subjective variables such as degree of ascites or encephalopathy [16]. The MELD score ranges from 6 to 40 and has been demonstrated to have prognostic value for survival in patients with underlying chronic liver disease, regardless of the etiology. Importantly, neither of these scoring systems assess tumor involvement.
As compared with other solid tumor types, the TNM staging system is less commonly employed for HCC, as it does not account for liver dysfunction, a crucial variable when examining treatment options for individual patients. The 7th edition American Joint Committee on Cancer (AJCC) staging system defines the stages for HCC as follows (Table 15.2): Stage I as a solitary tumor, any size, without vascular invasion; Stage II as a solitary tumor with vascular invasion or multiple tumors but none >5 cm in size; Stage IIIA as multiple tumors with at least one >5 cm in size; Stage IIIB as one or more tumors of any size involving a major branch of the portal vein or hepatic veins; and Stage IIIC as tumor(s) with perforation of the visceral peritoneum or direct invasion of adjacent organs other than the gallbladder [18]. Any regional lymph node involvement or distant metastases is classified as Stage IV disease.
Table 15.2
American Joint Committee on Cancer (AJCC) TNM Staging for Hepatocellular Carcinoma (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Solitary tumor without vascular invasion |
T2 | Solitary tumor with vascular invasion or multiple tumors, none > 5 cm |
T3a | Multiple tumors, one or more > 5 cm |
T3b | Tumor(s), any size, involving major branch of portal vein or hepatic veins |
T4 | Tumor(s) with perforation of visceral peritoneum or direct invasion of adjacent organs other than the gallbladder |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage IIIA | T3a | N0 | M0 |
Stage IIIB | T3b | N0 | M0 |
Stage IIIC | T4 | N0 | M0 |
Stage IVA | Any T | N1 | M0 |
Stage IVB | Any T | Any N | M1 |
Several alternative staging systems have been proposed to better define the prognosis of patients with HCC and appropriately stratify patients for treatment. One of the more established clinical staging systems is the Barcelona Clinic Liver Cancer (BCLC) system [19]. The BCLC classification stratifies patients on the basis of hepatic function as represented by the CTP score, clinical Eastern Cooperative Oncology Group (ECOG) performance status, and tumor stage, which encompasses tumor size, number of lesions, presence of vascular invasion, and extrahepatic spread of disease. Subsequent updates to the BCLC classification scheme have incorporated additional evidence-based treatment recommendations [6, 20]. The widely adopted EASL/EORTC consensus guidelines for management of HCC follow the BCLC staging algorithm (Fig. 15.4).
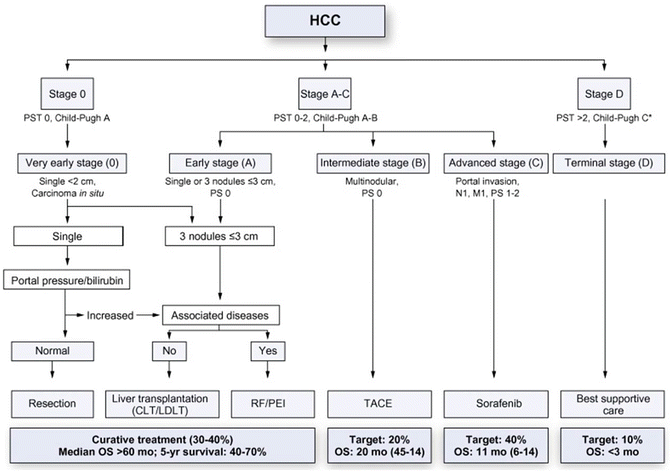

Fig. 15.4
BCLC (Barcelona Clinic Liver Cancer) staging system for management of HCC (Reprinted from European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56(4):908–43. With permission from Elsevier.)
Very early HCC (BCLC Stage 0) includes patients with an ECOG performance status 0; well-preserved liver function, defined as CTP Class A along with normal serum bilirubin and normal portal pressures; and a solitary HCC tumor, measuring less than 2 cm, with no evidence of vascular invasion. While few patients are typically diagnosed this early in their disease course, resection and transplantation both offer excellent 5-year survival rates of 80–90 % [21]. Early HCC (BCLC Stage A) includes patients with ECOG performance status 0, well-compensated CTP Class A liver disease, and solitary tumors >2 cm or up to three tumors, each <3 cm in diameter. For appropriately selected patients, 5-year survival approaches 50–70 % following hepatic resection or liver transplant [22].
Intermediate HCC (BCLC Stage B) includes patients with ECOG performance status 0, moderate liver dysfunction within CTP Class A or B, and large or multinodular tumors. As the majority of patients within BCLC Stage B are not surgical candidates for resection or transplant, locoregional therapy with chemoembolization generally offers the best chance for improved symptom control and survival within this cohort [19, 23].
Patients with advanced HCC (BCLC Stage C) include patients with diminished ECOG performance status, moderate liver disease within CTP Class A or B, and advanced tumors exhibiting macrovascular invasion and/or extrahepatic spread in the form of nodal disease or distant metastases. Stage C patients have a poor prognosis, and the multi-kinase inhibitor sorafenib (Onyx Pharmaceuticals, San Francisco, CA) is currently the only therapeutic option shown to have a survival benefit, demonstrating a 3-month improvement in overall survival as compared to placebo [24]. For patients without portal invasion or metastatic disease, locoregional liver-directed therapy with chemoembolization or radioembolization in addition to sorafenib can be considered.
Patients within BCLC Stage D include patients with extremely poor performance status (ECOG 3–4), advanced liver disease within CTP Class C, and advanced HCC. These patients have a terminal prognosis, with median survival of 3–4 months, and are treated with best supportive care and palliation [19].
Hepatic Resection
For patients with normal or minimally diseased underlying liver parenchyma and HCC amenable to surgical resection, liver resection remains the treatment of choice. Most patients, however, develop HCC in the setting of some degree of underlying liver disease or dysfunction, making appropriate patient selection for resection essential. Most patients with well-compensated CTP Class A cirrhosis can typically tolerate hepatic resection, while patients with Class C cirrhosis and nearly all patients with Class B cirrhosis are not candidates for resection. The presence of significant portal hypertension, the sequelae of which are typically detectable on preoperative imaging in the form of parenchymal changes, splenomegaly, and/or varices, is a risk factor for postoperative liver failure following resection. Low preoperative platelet count, another hallmark of portal hypertension, has also been shown to be an important independent risk factor for increased complications, postoperative liver insufficiency, and mortality following hepatic resection for HCC [25]. Pathologically, the degree of hepatic fibrosis can be quantified by the METAVIR scoring system, which assigns a score on a five-point scale from 0 to 4, ranging from no liver scarring to cirrhosis or advanced scarring [26]. This score in turn is predictive of liver’s ability to regenerate following hepatic resection.
A key consideration is the extent of the indicated hepatic resection, which must be balanced against the need to preserve an adequate functional liver remnant (FLR) with hepatic portal and arterial inflow, venous outflow, and biliary drainage [27]. The volume of the FLR (ideally >30 % of the total liver volume for patients with normal liver parenchyma or >40 % for well-compensated patients with cirrhotic liver parenchyma) must be taken into account, particularly in the setting of underlying liver disease [27]. Hepatic resection in the setting of fibrosis or cirrhosis carries increased risk of hepatic insufficiency and perioperative complications; this risk increases with the extent of resection (Fig. 15.5a, b). Portal vein embolization is a potential option to induce hypertrophy and increase the size of the FLR in cases where preoperative volumetric calculations suggest an inadequate FLR will remain following partial hepatectomy.
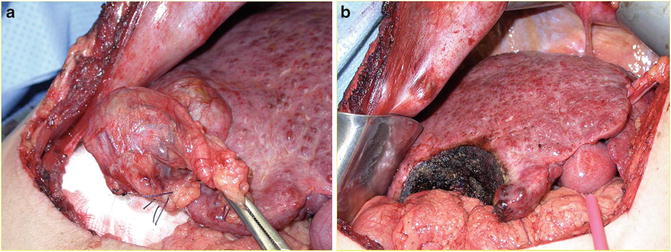

Fig. 15.5
(a) Hepatocellular carcinoma arising within a cirrhotic liver (note the fibrotic, nodular appearance of the uninvolved liver). (b) Liver remnant following limited hepatic resection of HCC lesion, again demonstrating the characteristic nodular appearance of cirrhosis
Ideal candidates for resection are patients with minimal or well-compensated liver dysfunction and unifocal, small lesions < 5 cm [7]. While multifocality and larger tumor size are not absolute contraindications for surgical resection, both features are surrogate markers for microscopic vascular invasion and more aggressive tumor histology [28]. Other tumor features associated with increased recurrence and worse survival include vascular invasion, infiltrative growth pattern, positive margin status, and lymph node involvement [29, 30]. In the absence of other adverse features, however, solitary tumors larger than 5 cm can be considered for resection if they involve < 50 % of the liver, as resection may offer 5-year survival rates of 20–25 % (Fig. 15.6a, b) [29, 30]. Resection margins of ≥2 cm are advocated when possible, as long as the adequacy of the FLR size is not compromised, as they are associated with improved recurrence-free and overall survival outcomes versus resection margins of 1 cm [31]. Techniques of resection are beyond the scope of this review, and we refer our readers to the following excellent sources:
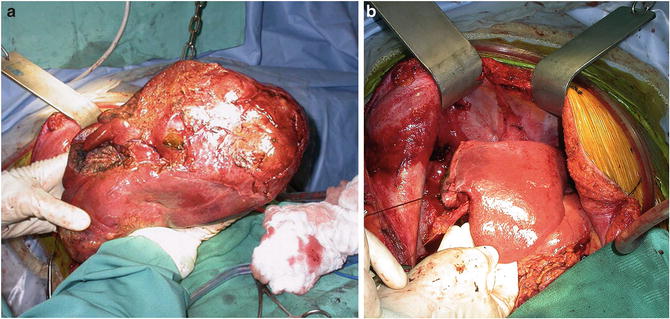

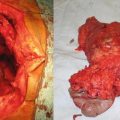
Fig. 15.6
(a) Intraoperative photograph of a large HCC lesion. (b) Liver remnant after resection of a large HCC lesion
Poon RT. Current techniques of liver transection. HPB. 2007; 9(3): 166–73.
Cunningham SC, Schulick RD. Management of Primary Malignant Liver Tumors. In: Cameron JL, Cameron AM (eds). Current Surgical Therapy, 10th edition. Philadelphia, PA: Elsevier Saunders; 2010.
Sicklick JK, D’Angelica M, Fong Y. The Liver. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL (eds). Sabiston Textbook of Surgery, 19th edition. Philadelphia, PA: Elsevier Saunders; 2012.
Fan ST. Major Hepatic Resection for Primary and Metastatic Tumors. In: Fischer JE (ed). Mastery of Surgery, 6th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
Maithel SK, Jarnagin WR, Belghiti J. Hepatic Resection for Benign Disease and for Liver and Biliary Tumors. Jarnagin WR (eds). Blumgart’s Surgery of the Liver, Biliary Tract, and Pancreas, 5th edition. Philadelphia, PA: Elsevier Saunders; 2012.
Patients with hepatitis B as the etiology of their cirrhosis and HCC often have comparatively well-preserved hepatic function versus patients with underlying hepatitis C, making resection a potentially more viable treatment for these patients. In Asia and Africa, where hepatitis B is endemic and where cadaveric organs are severely limited, resection is commonly employed for most patients with HCC amenable to surgical treatment. HCC arising secondary to NASH presents a new disease paradigm, and results to date suggest that these patients have a greater tendency to develop HCC within non-cirrhotic liver parenchyma. These patients may possess a theoretical lower risk of HCC recurrence in the liver remnant as compared with patients with underlying hepatitis B or C, and the benefit of resection may be greater in this patient population [32, 33].
One significant advantage of resection is the potential for immediate treatment, as opposed to the risk of disease progression while on the transplant wait list [34]. A trade-off for more expedited surgical therapy, however, is the significant risk of disease recurrence following partial hepatectomy. Recurrence rates following resection for HCC, whether from true recurrence or de novo tumor development in the cirrhotic liver remnant, have remained extremely high, reaching 50–75 % at 5 years in some series (Table 15.3) [35–47, 48]. Some groups have advocated a strategy of initial resection in patients with HCC within Milan Criteria and with relatively well-preserved liver function, followed by “salvage transplantation” or “secondary transplantation” for those who subsequently develop recurrent disease [49–52]. While primary resection of patients with early HCC and Child-Pugh Class A cirrhosis may be feasible, a significant portion of patients with recurrent disease following resection will not be candidates for transplantation, due to age, comorbidities, or recurrence outside of Milan Criteria [49, 51].
Table 15.3
Representative series of resected hepatocellular carcinoma from Western and Eastern series
Recurrence-free survival | Overall survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Author | Year | Study period | N | % Cirrhosis | Miscellaneous | 1 year | 3 years | 5 years | 1 year | 3 years | 5 years |
Llovet [35] | 1999 | 1989–1997 | 77 | 100 % | Mean size 3.3 cm | 73 % | 39 % | 25 % | 85 % | 62 % | 51 % |
Poon [36] | 2001 | 1989–1994 | 136 | 50 % | 72 % major resections | 42 % | 23 % | 16 % | 68 % | 47 % | 36 % |
1994–1999 | 241 | 43 % | 63 % major resections | 60 % | 38 % | 25 % | 82 % | 62 % | 49 % | ||
Belghiti [37] | 2002 | 1990–1999 | 328 | 50 % | 42 % major resections | NR | NR | NR | 61 % | 57 % | 37 % |
Ercolani [38] | 2003 | 1983–1999 | 224 | 100 % | Median size 4.1 cm | 70 % | 43 % | 27 % | 83 % | 63 % | 43 % |
Cha [39] | 2003 | 1990–2001 | 164 | 40 % | 85 % major resections | NR | NR | 25 % | 79 % | 51 % | 40 % |
Ikai [40] | 2007 | 1992–2003 | 27,062 | 43 % | 27 % multinodular | NR | NR | NR | 88 % | 69 % | 53 % |
Shah [41] | 2007 | 1992–2004 | 193 | 95 % | Median size 4.5 cm | 72 % | 48 % | 39 % | 85 % | 68 % | 53 % |
Park [42] | 2009 | 1994–2007 | 213 | 100 % | All within MC | 79 % | 57 % | 44 % | 92 % | 78 % | 69 % |
Dahiya [43] | 2010 | 1983–2002 | 373 | 100 % | 69 % major resections | 70 % | 43 % | 32 % | 82 % | 63 % | 44 % |
31 % minor resections (all tumors < 5 cm) | 67 % | 43 % | 32 % | 86 % | 65 % | 51 % | |||||
Lee [44] | 2010 | 1997–2007 | 130 | 100 % | 63 % within MC | 68 % | 54 % | 50 % | 80 % | 65 % | 52 % |
Huang [45] | 2011 | 2000–2005 | 648 | 100 % | Mean size 3.6 cm | 80 % | 57 % | 43 % | 94 % | 83 % | 76 % |
Arnaoutakis [46] | 2013 | 1992–2011 | 334 | 0 % | Median size 6.5 cm | 71 % | NR | 35 % | 87 % | NR | 55 % |
Sapisochin [47] | 2013 | 1991–2007 | 95 | 100 % | All <5 cm in size | 81 % | NR | 33 % | 85 % | NR | 62 % (4 years) |
Transplantation
Liver transplant offers arguably the most effective cure for HCC, as it removes both the malignancy and the underlying diseased liver parenchyma in which HCC typically arises. Transplantation is limited, however, by access to donor organs and must be balanced against the need for lifelong immunosuppression. Across the globe, the most widely accepted transplant selection criteria are referred to as the Milan Criteria. First reported in 1996 by Mazzaferro et al [53], the Milan Criteria defined transplant criteria for patients as a single HCC lesion < 5 cm in maximum diameter or ≤ 3 lesions each < 3 cm in size, with no evidence of macrovascular invasion or extrahepatic disease on imaging. Numerous studies worldwide, many included in a comprehensive 2011 meta-analysis by the Milan group, have confirmed the favorable outcomes that can be achieved with transplantation for patients meeting these criteria [54]. Others have advocated broader transplantation guidelines, such as the expanded University of California, San Francisco (UCSF) criteria, which include patients with a single lesion < 6.5 cm or up to three tumors, each measuring less than 4.5 cm and a total tumor diameter < 8 cm [55, 56].
As a result of limited organ availability, the drawbacks of transplantation include the risk for disease progression and resulting patient dropout, while patients are on the transplant wait list. Particularly in parts of Asia where HCC is more prevalent, the number of patients with HCC on transplant wait lists far exceeds the supply of deceased donor livers available. In the United States, the UNOS (United Network for Organ Sharing) criteria dictate that patients with HCC meeting Milan Criteria radiographically receive a MELD “exception points” score of 22 when placed on the wait list. If patients remain on the wait list after 3 months, they typically receive an additional three exception points. Within this allocation scheme, wait times vary considerably across UNOS regions and globally, with median times to transplant of 6–12 months in many regions increasing patient dropout and affecting intention-to-treat outcomes [34, 57, 58]. As a result, many centers, particularly those with longer wait times, now offer locoregional neoadjuvant or “bridging” therapy to patients on the transplant wait list to attempt to minimize tumor progression while awaiting a donor organ [59]. Several non-randomized studies to date have reported decreased dropout rates, but none have demonstrated a correlation between pre-transplant bridging therapy with ablation or transarterial chemoembolization and improved posttransplant survival [60–64]. A cost-effective analysis of pretransplantation bridging ablation therapy, however, demonstrated benefit if projected wait time to transplant exceeded 6 months [65].
Downstaging
No randomized controlled trials have evaluated the utility of locoregional therapy for downstaging patients initially outside of Milan Criteria, although several small series have demonstrated comparable 5-year outcomes for such patients successfully treated with radiofrequency ablation or chemoembolization followed by transplantation versus patients who meet Milan Criteria a priori [66–68]. In light of limited donor organ availability, studies are ongoing to better define which patients beyond Milan Criteria are most likely to benefit from downstaging followed by transplantation.
Living Donor Liver Transplantation
Living donor liver transplantation (LDLT) is also an option for patients and avoids the potential limitations of wait times for allocation of deceased donor livers and the restrictions of the Milan Criteria, although LDLT has been slow to be adopted. Some concerns were raised by early studies suggesting patients undergoing LDLT for treatment of HCC had higher rates of recurrence than seen with deceased donor transplantation [69, 70], although overall survival outcomes appear comparable [71, 72]. Because wait times are minimized, patients with more aggressive tumors that would progress and render them ineligible for deceased donor transplant may be undergoing LDLT; thus, an observation period of 2–3 months has been proposed to assess the natural history of a patient’s tumor [69, 73]. Markov cost-effectiveness modeling suggests that LDLT is most cost-effective in scenarios where wait list times are projected to exceed 7 months [74].
Locoregional Therapy
Ablation
Local ablation is the treatment of choice for patients with early-stage HCC not amenable to surgical therapies. Modalities include radiofrequency, chemical, and microwave ablation.
Radiofrequency ablation (RFA) involves the delivery of electrical energy to cause coagulative necrosis of tumor tissue and can be performed percutaneously, laparoscopically, or as an adjunct procedure from an open surgical approach. One recent study of early HCC lesions < 2 cm demonstrated sustained complete response in 95 % of patients following ablation, with a local recurrence rate of < 1 % [75]. For tumors >3 cm, the efficacy of ablation diminishes significantly [76]. Only two randomized, controlled comparisons of ablation versus resection for early HCC have been performed to date, with one study demonstrating equivalent recurrence and survival rates for the two treatment modalities and the other study suggesting resection was associated with lower recurrence rates and improved survival compared to RFA [77, 78]. Thus the use of RFA as a first-line definitive therapy in patients with resectable disease is not widely practiced. For patients who are not candidates for resection, however, ablation offers an excellent treatment option for smaller tumors. RFA also can be employed as a bridging therapy for HCC in patients awaiting liver transplantation [79].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree