SUMMARY
In patients with acute liver failure or chronic liver disease, many changes in the hemostatic system occur. The liver is the site of synthesis of nearly all coagulation factors, both pro- and anticoagulant proteins. A reduced synthesis function of the liver will lead to reduced levels of these factors in circulation. In addition, the liver is involved in the clearance of many activated coagulation factors and protein–inhibitor complexes from the circulation, which, in turn, can lead to activation of the coagulation system if liver function is impaired. Furthermore, the liver is involved in the synthesis and clearance of pro- and antifibrinolytic proteins, which may lead to a shift in the balance of the fibrinolytic system. Also primary hemostasis might be affected in liver disease because of thrombocytopenia and impaired platelet function, which is frequently encountered in these patients. It is evident that patients with liver disease have frequent bleeding episodes, mainly in the gastrointestinal tract, such as variceal bleeding. It has been a longstanding dogma that patients with liver disease are at a high risk of bleeding caused by the above mentioned hemostatic changes. However, in recent years, this cause of the bleeding tendency has been questioned because of the concomitant reductions of pro- and anticoagulant factors and pro- and antifibrinolytic factors. More recent studies using more sophisticated coagulation tests showed that thrombin generation is normal in patients with chronic liver failure and that some may even have a prothrombotic phenotype. This led to the development of a model of a rebalanced hemostatic system in these patients, which may have immediate implications for treatment. Hematologists and other clinicians taking care of patients with acute liver failure of chronic liver disease, such as cirrhosis, are still faced with the questions of whether these patients need correction of the changes in hemostasis before interventions such as paracentesis, biopsies, dental care, and surgery. It was generally believed that replacement therapy with frozen plasma or prothrombin complex concentrate was indicated. However, based on these new findings, physicians should now be more restrictive in the use of hemostatic agents and blood products in these patients both in liver disease and during liver transplantation.
Acronyms and Abbreviations:
ADAMTS13, a disintegrin-like and metalloprotease with thrombospondin domain 13; aPTT, activated partial thromboplastin time; DDAVP, 1-deamino-8-D-arginine vasopressin; DIC, disseminated intravascular coagulation; FFP, fresh-frozen plasma; HAT, hepatic artery thrombosis; HSC, hepatic stellate cell; INR, international normalized ratio; ISI, international sensitivity index; LMWH, low-molecular-weight heparin; MELD, model of end-stage liver disease; PAI-1, plasminogen activator inhibitor 1; PFA, platelet function analyzer; PT, prothrombin time; PVT, portal vein thrombosis; TAFI, thrombin-activatable fibrinolysis inhibitor; t-PA, tissue-type plasminogen activator; VWF, von Willebrand factor.
The liver plays a central role in the hemostatic system. Liver parenchymal cells are the site of synthesis of most coagulation factors (except factor VIII), the natural inhibitors of coagulation, including protein C, protein S, and antithrombin, and essential components of the fibrinolytic system, such as plasminogen, α2-antiplasmin, and thrombin activatable fibrinolysis inhibitor (TAFI). The liver also regulates hemostasis and fibrinolysis by clearing activated coagulation factors and coagulation factor–inhibitor complexes from the circulation. In addition, changes in primary hemostasis mediated by platelets, von Willebrand factor (VWF), and ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type 1 repeats) may occur. Therefore, when acute or chronic liver dysfunction is present in patients with liver disease, complicated hemostatic derangement may occur, which can lead to bleeding, thrombosis, or neither bleeding nor thrombosis.
More than 75 percent of patients with chronic liver disease, especially in moderate to severe cirrhosis (Child B and C), have reduced levels of platelets (<150,000/μL), and 13 percent have platelet counts between 50,000 and 75,000/μL.1 This may be caused by splenomegaly resulting in sequestration of platelets in the spleen, reduced synthesis of thrombopoietin by the diseased liver, or consumption coagulopathy.2–5 In addition, it has been suggested that autoantibodies against platelets may reduce the half-life of platelets in cirrhosis.6 Primary hemostasis may also be defective by a reduced platelet function. In vitro platelet aggregation studies in response to various agonists are frequently diminished in patients with liver disease. Defective platelet function may result from impaired signal transduction, acquired storage pool deficiency, proteolysis of platelet membrane proteins, and increased production of the endothelial-derived platelet inhibitors, nitric oxide and prostacyclin (reviewed in Ref. 7). A reduced hematocrit may contribute to defective platelet–vessel wall interaction. Platelet adhesion defects were also found under conditions of flow, but were in some studies attributed to thrombocytopenia and a low hematocrit.8–10 Platelet procoagulant activity measured by a thrombin generation assay using platelet-rich plasma was similar in patients and healthy controls, which casts additional doubt on the extent of the functional defects of platelets in patients with liver disease.11
VWF antigen levels are strongly elevated in patients with liver disease. It has been suggested that this is the result of endothelial damage possibly mediated by endotoxemia (bacterial infection).12,13 VWF mRNA and protein expression in the liver itself are substantially increased in cirrhosis, but VWF ristocetin cofactor activity is variable.10,14–16 The high levels of VWF may ameliorate the hemostatic defect caused by thrombocytopenia and platelet function defects.13 In a flow-based model, platelet adhesion to collagen was normalized in thrombocytopenia because of the high levels of VWF in cirrhotic plasma. In patients with liver disease, the regulation of VWF multimer size and activity can be impaired because of reduced synthesis of the VWF-cleaving protease ADAMTS13 by stellate cells in the liver.17 Several studies showed, however, that the most active high-molecular-weight multimers of VWF are diminished in plasma of patients with cirrhosis, which may be mediated by plasmin or other proteases.18,19 Classical tests of primary hemostasis, such as bleeding time, which is becoming obsolete as a result of its assay variability and inability to predict bleeding, may still be abnormal in patients with liver disease. Also, newer global tests of primary hemostasis, such as platelet function analyzer (PFA), show prolonged closure times with various agonists, but its value in prediction of bleeding in liver disease is unknown.20
The liver is the site of synthesis of most procoagulant proteins. As a result, decreased levels of coagulation factors II, V, VII, IX, X, and XI are commonly observed in patients with liver failure.21 In contrast, factor VIII levels are increased, which may be related to the elevated level of its carrier protein VWF and to decreased clearance of factor VIII from the circulation by the liver low-density lipoprotein-related receptor.14 Factor VIII is synthesized primarily in hepatic sinusoidal endothelial cells, whose function is preserved in liver disease.14,22 Acquired vitamin K–dependent carboxylation deficiency may lead to qualitative defects in coagulation factors. Because of vitamin K deficiency or decreased production of γ-glutamyl carboxylase, circulating vitamin K–dependent coagulation factors II, VII, IX, and X may be deficient in γ-carboxylated glutamic acid residues in their GLA domains, giving rise to impaired function of these factors.23 On the other hand, levels of anticoagulant protein C, protein S, antithrombin, heparin cofactor II, and α2-macroglobulin are also decreased in patients with liver disease.24 Fibrinogen levels are frequently in the normal range in patients with chronic liver disease, but may be decreased in patients with decompensated cirrhosis or acute liver failure.25 A qualitative defect in fibrinogen may be found in patients with liver disease.26 Screening tests of coagulation, such as the prothrombin time (PT) or activated partial thromboplastin time (aPTT), are frequently prolonged in patients with chronic liver disease. These results have been traditionally interpreted to reflect a hypocoagulable state. The PT and aPTT are sensitive to levels of procoagulant proteins in plasma, but not to the natural anticoagulants, protein C, protein S, and antithrombin. The use of a more sophisticated test of coagulation, such as total thrombin generation test, illustrates the limitation of PT and aPTT. In a thrombin-generation test measuring the total amount of thrombin generated during coagulation, decreased total thrombin generation is measured in patients with cirrhosis compared to controls.11,27,28 Yet, when measured in the presence of thrombomodulin to enable protein C activation and thereby also taking into account the contribution of the main inhibitor of coagulation protein C, thrombin generation was indistinguishable from controls, despite abnormal conventional coagulation tests. Others found normal thrombin generation without addition of thrombomodulin and even increased thrombin generation with addition of thrombomodulin.29,30 These results suggest that thrombin generation in vivo can be normal in patients with liver failure and that a prolonged PT does not per se indicate a bleeding risk. These findings indicate that a concomitant decrease of pro- and anticoagulant factors results in a rebalanced hemostatic system.31
Despite the limitations of the use of PT in patients with liver disease, the international normalized ratio (INR), which is a derivative of PT, is still used in prognostic scores for patients with acute or chronic liver disease. The model of end-stage liver disease (MELD) score is used to prioritize patients for liver transplantation. The INR was originally developed and validated only to monitor anticoagulant therapy with vitamin K antagonists. The interlaboratory variation of the INR in patients with liver disease is substantial, and its use results in significant differences in MELD scores when a single patient sample is tested in different laboratories using various PT reagents.32,33 The use of alternative international sensitivity index (ISI) values obtained by calibration against plasma samples from patients with liver disease was shown to decrease this variability.34,35
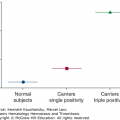
Except for tissue-type plasminogen activator (t-PA) and plasminogen-activator inhibitor (PAI)-1, all proteins involved in fibrinolysis, both pro- and antifibrinolytic, are synthesized by the liver.36 Therefore, chronic liver disease leads to decreased plasma levels of plasminogen, α2-antiplasmin, TAFI, and factor XIII. Plasma levels of t-PA are elevated as a result of increased secretion from endothelial cells and/or reduced clearance by the diseased liver. Plasma levels of PAI-1 also are increased but not to the same extent as t-PA, which may lead to a shift in balance in the fibrinolytic system.37 It has long been assumed that most patients with chronic liver disease had accelerated fibrinolysis. This was based on in vitro assays, including various clot lysis assays, and on measurements of increased fibrin(ogen) degradation products, D-dimer, and plasmin–antiplasmin complexes (reviewed in Ref. 36). However, more recent studies found no evidence of hyperfibrinolysis in the majority of patients with cirrhosis despite decreased levels of TAFI and elevated D-dimer levels.38,39 This conclusion was recently challenged by a study that used two assays to detect fibrinolysis in patients with various degrees of severity of cirrhosis. In both tests, approximately 40 percent of patients had evidence of hyperfibrinolysis, and in 60 percent of the patients, one of the tests revealed an increased fibrinolytic capacity, especially in those with severe liver dysfunction.40 Hyperfibrinolysis in patients with cirrhosis may also occur secondary to low-grade disseminated intravascular coagulation (DIC) induced by endotoxemia and is manifested by concomitant increased levels of prothrombin fragment 1+2, fibrinopeptide A, D-dimer, thrombin–antithrombin complex, and plasmin–antiplasmin complex.41 However, it has been argued that the increased levels of these markers may result from their decreased clearance by the liver rather than from DIC. In patients with liver disease who presented with gastrointestinal bleeding or soft tissue bleeding after trauma, in vitro signs of increased fibrinolysis have been reported.42,43
It has been a longstanding dogma that patients with liver disease are at a high risk of bleeding due to reduction of synthesis of coagulation factors and other changes in hemostasis. More recent studies using more sophisticated coagulation tests have shown that thrombin generation is normal in patients with chronic liver failure and that some may even have a prothrombotic phenotype.24,27,44 Because both procoagulant and anticoagulant proteins decline in patients with chronic liver diseases, it has been postulated that the hemostatic system is rebalanced (Table 18–1).24,31,45 In addition, reductions of platelet number and impairment of platelet function are counteracted by high levels of VWF, and in many patients, the decline in profibrinolytic factors is balanced by the reduction of inhibitors of fibrinolysis.13,38 This led to the model of a rebalanced hemostatic system in these patients, which has important implications for treatment.24,31 This model also explains why most patients with liver disease usually do not exhibit severe bleeding manifestations—neither during minor invasive procedures, such as biopsies and paracentesis, nor during major surgeries, including liver transplantation.46,47 Furthermore, patients with liver disease may even have increased risk of venous thromboembolism, not only liver-specific thrombosis, but also deep vein thrombosis.48–50 The hemostatic balance in patients with liver disease is, however, quite delicate and vulnerable to be tipped toward bleeding or thrombosis. So far, it is impossible to identify which patients are more prone to bleeding or to thrombosis based on current laboratory assays. The complex changes in hemostasis encountered in patients with liver disease are depicted in Table 18–1. The delicate hemostatic balance in patients with liver disease may be changed by comorbidities, such as bacterial infections and renal failure frequently observed in these patients. It is of major importance to treat these comorbidities so as to reduce the risk of bleeding and thrombosis.51
| Changes That Impair Hemostasis | Changes That Promote Hemostasis |
|---|---|
| Primary Hemostasis | |
| Thrombocytopenia | Elevated levels of VWF |
| Platelet function defects | Decreased levels of ADAMTS13 |
| Enhanced production of nitric oxide and prostacyclin | |
| Secondary Hemostasis | |
| Low levels of factors II, V, VII, IX, X, and XI | Elevated levels of factor VIII |
| Vitamin K deficiency | Decreased levels of protein C, protein S, antithrombin, α2-macroglobulin, and heparin cofactor II |
| Dysfibrinogenemia | |
| Fibrinolysis | |
| Low levels of α2-antiplasmin, factor XIII, and TAFI | Low levels of plasminogen Increase in PAI-1 levels |
| Elevated t-PA levels |
Patients presenting with acute liver failure, for instance in acetaminophen intoxication, have profound changes in the hemostatic system. A severe decrease of coagulation factors is observed, with strongly increased INR.52 However, an intact thrombin generation has been observed in acute liver failure patients, and hardly any changes were observed using thromboelastography.53,54 In contrast to chronic liver disease, patients with acute liver failure frequently have normal platelet counts. Highly elevated levels of VWF and strongly decreased levels of ADAMTS13 are observed. This imbalance may lead to a prothrombotic state.55 In patients with acute liver failure, there is an increased level of PAI-1 and reduced levels of plasminogen, which is consistent with a hypofibrinolytic state.53,56 A strong increase of procoagulant microparticles has been observed in acute liver failure.57 Spontaneous bleeding is not frequently encountered in patients with acute liver failure.58
Liver transplantation performed in patients in acute or chronic liver failure has always been complicated by significant and sometimes life-threatening bleeding problems requiring massive use of coagulation factors and erythrocyte transfusion.59 Therefore, blood products were also transfused before and during transplantation to correct the hemostatic dysfunction. Improved surgical techniques and anesthesiologic care have led to a remarkable reduction of blood loss during liver transplantation. Currently, no blood transfusion is given in up to 50 to 80 percent of patients undergoing a liver transplantation, depending on the center.60,61 This improvement is also because of a better understanding of the coagulation profile during the various stages of the surgical intervention. During the first stage of liver transplantation, the removal of the diseased liver, no significant worsening of the preoperative hemostatic status occurs.62 After removal of the diseased liver, the so-called anhepatic stage, significant hemostatic changes occur. Because activated coagulation factors are not cleared from the circulation, system activation of coagulation, in its most extreme form mimicking DIC, can develop, with consumption of platelets and coagulation factors and secondary hyperfibrinolysis. Moreover, hyperfibrinolysis may also occur as a result of defective clearance of t-PA.63 The most severe hemostatic changes during liver transplantation occur immediately after reperfusion of the donor liver. Platelets are trapped in the graft, giving rise to an aggravation of thrombocytopenia and causing damage to the graft by induction of endothelial cell apoptosis.64 Release of tissue factor and t-PA from the reperfused graft causes DIC with primary or secondary fibrinolysis.63 Moreover, the graft also releases heparin-like substances that can inhibit coagulation.65 In addition, other factors such as hypothermia, metabolic acidosis, and hemodilution adversely affect hemostasis during this phase.
During transplantation, the balance between VWF and ADAMTS13 changes because levels of VWF remain high, the functional properties of VWF improve, and the levels of ADAMTS13 decline (mostly due to impaired synthesis and possibly due to enhanced proteolytic degradation), which may partially compensate for the hemostatic dysfunction.66 The platelet count and hemostatic proteins are at their nadir after reperfusion and rise gradually during the early postoperative period. However, the levels of procoagulant factors rise more rapidly than the levels of anticoagulant factors, which results in a temporary hypercoagulable state.67 A transiently increased level of PAI-1 immediately after surgery can result in a hypofibrinolytic state that may aggravate the hypercoagulable status.
CLINICAL PROBLEMS ENCOUNTERED IN PATIENTS WITH LIVER DISEASE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree