Hemoptysis
Randolph J. Lipchik
Hemoptysis, the coughing or expectoration of blood that originates in the lung, can be an alarming symptom for both patient and physician. It can range from blood-tinged or streaked sputum to massive hemoptysis, the latter defined as blood loss of 400–600 mL per day. Massive hemoptysis occurs in fewer than 5% of cases but carries a mortality rate of up to 85% if surgical intervention is not feasible (1, 2). The management of hemoptysis, therefore, requires careful consideration of the cause, severity of the process, and functional status of the patient. Management may be more aggressive and invasive early in the course of a malignancy, whereas this could be inappropriate or dangerous for a patient in the terminal stages of an illness.
Pathogenesis
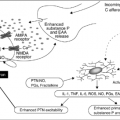
The lung is perfused by two distinct circulations that must be considered when determining the source and planned treatment of hemoptysis. The pulmonary circulation delivers blood under low pressure from the right ventricle to the alveolar capillaries for exchange of oxygen and carbon dioxide. The bronchial circulation, which is approximately 1–2% of the cardiac output, arises from the systemic circulation and provides nutrient flow to the lung parenchyma. A detailed review of the anatomy and physiology of the bronchial circulation has been published (3) and is beyond the scope of this chapter. In brief, two or more arteries arise from the aorta or upper intercostal arteries, enter the lung, and eventually form a plexus, which accompanies the branching airways with small, penetrating arteries, forming another plexus that supplies the bronchial mucosa down to the terminal bronchioles. Farther on, they anastomose with both precapillary pulmonary arterioles and pulmonary veins. Bronchial venous return is more complex: Veins from the proximal airways return blood to the right atrium through the azygous, homozygous, or intercostal veins, whereas the intrapulmonary bronchial venous blood returns through the pulmonary veins to the left ventricle. The latter occurs because of anastomoses between bronchial and pulmonary veins and carries the bulk of bronchial venous return. Although the bronchial circulation is nonessential in the normal adult lung, in the setting of chronic inflammation, neoplasm, or repair after lung injury, bronchial blood flow increases because of increases in both the size and number of vessels. Elevations of pulmonary vascular pressure can affect the bronchial circulation because of the many anastomoses between the dual circulations. In general, hemoptysis occurs because of disruption of the high-pressure bronchial vessels, which become abnormally enlarged and exposed within diseased airways.
Etiology
This discussion concentrates on the malignant causes of hemoptysis, but awareness of other causes is important because many patients have underlying conditions that may become active problems during treatment of a malignant disease. The differential diagnosis for a patient presenting with hemoptysis is extensive (Table 26.1). Some conditions that are more likely to be associated with massive hemoptysis are shown in Table 26.2. In past years, tuberculosis, bronchiectasis, and lung abscess were the most common causes of massive hemoptysis. The incidence of the latter two has declined in industrialized nations, but tuberculosis remains a significant problem worldwide. Experience with tuberculosis has helped us to understand the pathophysiology of massive hemoptysis. Up to 7% of deaths from tuberculosis have been attributed to massive hemoptysis; autopsy examinations have revealed ruptured pulmonary artery aneurysms. Rasmussen has described localized ruptures of aneurysmal portions of pulmonary arteries passing through thick-walled cavities of chronic tuberculosis, the so-called “Rasmussen’s aneurysms.” Rupture occurs secondary to the infection or the associated inflammatory response (4). Healed calcified mediastinal lymph nodes from prior tuberculosis can erode into the bronchial mucosa, also causing significant bleeding. Tuberculosis distorts lung architecture, causing bronchiectasis with resulting hypertrophy and proliferation of bronchial vessels. Infection or inflammation in these diseased portions of the airway can cause rupture of vessels; the result is massive bleeding caused by the high systemic arterial pressure.
Table 26.1 Causes Of Hemoptysis | ||||
|---|---|---|---|---|
| ||||
Table 26.2 Causes of Massive Hemoptysis | |
|---|---|
|
Nonmalignant Conditions
Patients with preexisting cavitary lung disease resulting from mycobacterial infections, sarcoidosis, bullous emphysema, lung abscess, lung infarction, and fibrocavitary disease secondary to rheumatoid disease are at risk for mycetoma formation, most often due to Aspergillus. This noninvasive infection results in a thick-walled cavity with vascular granulation tissue and inflammatory cells, the former the result of proliferation of the bronchial circulation. Bleeding is a result of vascular injury from fungal endotoxin, proteolytic activity, or a type 3 hypersensitivity reaction (5). Bacterial superinfection also can promote hemoptysis in the setting.
Deep venous thrombosis and the subsequent thromboembolic disease are common in hospitalized patients, especially those with underlying risk factors. The presence of a malignancy is a major risk factor, and the onset of hemoptysis
warrants consideration of the possibility of pulmonary embolism and subsequent infarction. With current standard anticoagulation therapy, pulmonary embolism is a treatable condition with a 2.5% mortality rate. In a prospective study of the clinical course of pulmonary embolism, almost 24% of patients died within 1 year of diagnosis. Many cases, approximately 35%, had some form of cancer (6). A less-well-appreciated and studied source of pulmonary embolism is upper extremity thrombosis resulting from indwelling venous catheters. In some series, the incidence of central line thromboembolism has been as high as 12% (7). Although most commonly seen in the pediatric population, there have been reports of inhaled foreign bodies in adults that, if unrecognized, have caused hemoptysis (8).
warrants consideration of the possibility of pulmonary embolism and subsequent infarction. With current standard anticoagulation therapy, pulmonary embolism is a treatable condition with a 2.5% mortality rate. In a prospective study of the clinical course of pulmonary embolism, almost 24% of patients died within 1 year of diagnosis. Many cases, approximately 35%, had some form of cancer (6). A less-well-appreciated and studied source of pulmonary embolism is upper extremity thrombosis resulting from indwelling venous catheters. In some series, the incidence of central line thromboembolism has been as high as 12% (7). Although most commonly seen in the pediatric population, there have been reports of inhaled foreign bodies in adults that, if unrecognized, have caused hemoptysis (8).
Malignant Conditions
In a retrospective review of 877 cases of lung cancer, Miller and McGregor reported a 19.3% overall incidence of hemoptysis, with non-life-threatening hemoptysis occurring equally among histologic types (9). Twenty-nine cases (3.3%) were massive and terminal events, due almost exclusively to proximal, cavitary squamous cell carcinomas. In only 6 of these 29 cases was there no antecedent nonlethal bleeding. The cause of this sudden catastrophic bleeding was tumor hemorrhage or invasion of a pulmonary artery or vein. In another series, Panos et al. (10) also found an association between cavitary squamous cell tumors and fatal hemoptysis. Metastatic endobronchial disease (carcinoma of breast, colon, kidney, and melanoma) is more likely to cause nonfatal hemoptysis rather than a terminal bleeding event. The incidence of hemoptysis in patients with bronchial carcinoid tumors approaches 50%, resulting from mucosal ulceration or airway inflammation disrupting the bronchial arteries supplying these tumors. The high incidence of symptomatic bleeding is not surprising, as 85% of carcinoids arise in the proximal airway and are often very vascular (11). Malignant tracheal tumors are uncommon; when present, they usually result in obstructive symptoms. Hemoptysis does occur from these tumors, but less frequently than with bronchogenic carcinoma (12). A Danish series of pulmonary hamartoma cases found that only 39% of patients were symptomatic but nearly one fourth of those patients noted hemoptysis (13).
Patients with a hematologic malignancy may develop hemoptysis for many reasons, including thrombocytopenia, coagulation abnormalities, and infections. In one series, fatal hemoptysis was associated strongly with the autopsy
findings of vascular invasion, thrombosis, and hemorrhagic infarction secondary to invasive fungal disease (14). Idiopathic alveolar hemorrhage is a rare cause of fatal hemoptysis, accounting for only 2–3% of leukemia deaths, but it also is associated with nonfatal hemoptysis. This is hypothesized to be attributable to the combination of thrombocytopenia and diffuse alveolar damage, the latter a result of chemotherapy, radiotherapy, sepsis, viral infection, or a combination of any of these (14).
findings of vascular invasion, thrombosis, and hemorrhagic infarction secondary to invasive fungal disease (14). Idiopathic alveolar hemorrhage is a rare cause of fatal hemoptysis, accounting for only 2–3% of leukemia deaths, but it also is associated with nonfatal hemoptysis. This is hypothesized to be attributable to the combination of thrombocytopenia and diffuse alveolar damage, the latter a result of chemotherapy, radiotherapy, sepsis, viral infection, or a combination of any of these (14).
Thromboembolic disease already has been discussed in this chapter, but pulmonary embolic disease may also be caused by intravascular tumor metastases, resulting in a clinical presentation indistinguishable from the more common venous thromboembolism. Hemoptysis is unusual, and symptoms of dyspnea and right heart failure predominate. Rarely, massive tumor embolism results in pulmonary infarction with hemoptysis. Pulmonary infarction due to malignant compression of pulmonary veins also has been reported (15).
Diagnosis
The key element of diagnosis in cases of hemoptysis is the localization of bleeding to the lower respiratory tract. Although blood from the stomach usually has a low pH and blood from the respiratory tract a high pH, bleeding from the nasopharynx, larynx, or gastrointestinal tract may be difficult to distinguish clinically from true hemoptysis. Furthermore, bleeding from these sources may result in cough and the appearance of blood, which can be misinterpreted as hemoptysis. When there is doubt, a thorough examination of the nasopharynx, larynx, and upper gastrointestinal tract should be performed.
Once the lung has been identified as the source of bleeding, the next step is to localize the site of bleeding. Physical examination alone is not sensitive enough; a chest x-ray should be performed, and is often helpful in revealing a tumor or abscess. However, it can be misleading, as blood may be coughed into uninvolved portions of the lungs. Bronchoscopy is the surest way to visualize the source (or at least the segment) from which there is active bleeding. Flexible fiberoptic bronchoscopy is usually attempted first because it can be done relatively quickly at the bedside without general anesthesia, and can access more distal airways than the rigid bronchoscope. The latter has the advantages of greater suction capability, removal of clots or foreign bodies, and airway control that allows for patient ventilation, often necessary in cases of massive hemoptysis. Computed tomography (CT) scan has been compared with bronchoscopy in studies in which hemoptysis is the presenting problem. Patients with preexisting cancer constituted a small proportion of those studied. The CT scan is superior in identifying bronchiectasis, lung abscess, aspergilloma, and distal parenchymal abnormalities; however, the bronchoscope can obtain material that allows cytologic, histologic, and microbiologic diagnoses (16, 17). In a recent study, it was suggested that CT may be the more efficient diagnostic tool (18). In patients with established malignancies, a CT scan may offer important information, as it can delineate peribronchial or mediastinal involvement of tumors that cannot be seen with a bronchoscope. Routine use of CT scan is not of proven benefit but should be considered in cases in which the chest radiographic findings are inconclusive, or to provide a more detailed anatomic localization of an abnormality for the bronchoscopist.
Management
The severity of hemoptysis determines the pace at which a workup should proceed. In a review of 10 years’ experience at Duke University Medical Center, the mortality rate was 9% and 58% if blood loss was less than or more than 1000 mL per 24-hour period, respectively (1). A malignant cause for hemoptysis of greater than 1000 mL per 24 hours increased the mortality rate to 80%. Massive hemoptysis requires rapid intervention to guarantee that the patient has an adequate airway while attempting to control bleeding. If blood loss is minimal and sporadic, a more detailed evaluation can occur without immediate attention to resuscitative efforts. Early consultation with a pulmonary physician and thoracic surgeon is recommended.
Initial diagnostic studies should include chest radiograph, hematocrit, platelet count, blood urea nitrogen, serum creatinine, and coagulation panel. Oxygenation should be monitored by arterial blood gas determination or pulse oximetry, and adequate intravenous access established. Typed and cross-matched blood should be available in cases of significant bleeding. Mild sedation and judicious use of a cough suppressant can be employed, but excessive use compromises a patient’s ability to clear the airway.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree