Hemochromatosis
![]()
EPIDEMIOLOGY
Classic hereditary hemochromatosis, also known as HFE-hemochromatosis, is an autosomal recessive disorder caused by inappropriate dietary absorption of iron and abnormal iron cycling. It is characterized by progressive accumulation of iron in tissues, particularly the liver, pancreas, heart, endocrine organs, and skin, which may lead to end-stage organ damage, usually during or after middle age.1–3 It is one of the most common single-gene disorders in Caucasians of northern European descent, with an incidence of 1 in 200 and a carrier rate of 1 in 10 persons. However, the clinical penetrance of the disorder is highly variable, and only a minority of affected persons develops severe or life-threatening organ dysfunction.4,5
Genetic Basis for Classic Hemochromatosis: HFE Mutations
![]() Mutations in HFE, an MHC class-I like gene on chromosome 6, are found in nearly 90% of people with the clinical phenotype and 100% of affected people with a strong family history of the disorder. 6,7
Mutations in HFE, an MHC class-I like gene on chromosome 6, are found in nearly 90% of people with the clinical phenotype and 100% of affected people with a strong family history of the disorder. 6,7
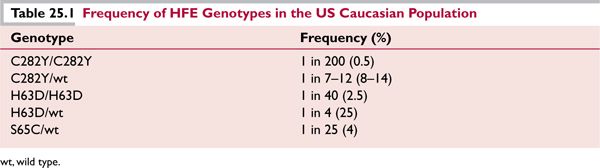
![]() Substitution of tyrosine for cysteine at amino acid 282 of the HFE gene product (C282Y) is the founder mutation; by linkage disequilibrium analysis, the mutation originated recently, within the past 2000 years. C282Y occurs with highest frequency in northwestern European populations, reaching 14% in areas of Great Britain (Table 25.1). Allele frequency decreases in a north-to-south and west-to-east direction across Europe, and the ancestral haplotype may have been of Viking or Celtic origin; the haplotype is extremely rare in African and Asian populations. Homozygosity for C282Y is seen in 64% to 96% of persons with clinical hemochromatosis.
Substitution of tyrosine for cysteine at amino acid 282 of the HFE gene product (C282Y) is the founder mutation; by linkage disequilibrium analysis, the mutation originated recently, within the past 2000 years. C282Y occurs with highest frequency in northwestern European populations, reaching 14% in areas of Great Britain (Table 25.1). Allele frequency decreases in a north-to-south and west-to-east direction across Europe, and the ancestral haplotype may have been of Viking or Celtic origin; the haplotype is extremely rare in African and Asian populations. Homozygosity for C282Y is seen in 64% to 96% of persons with clinical hemochromatosis.
![]() A second HFE mutation, replacement of histidine by aspartate at residue 63 of the HFE protein (H63D) is frequently found on the non-C282Y-containing chromosome of individuals with clinical hemochromatosis who are heterozygous for C282Y.6 H63D is an older mutation with a wider population distribution, having an allele frequency of 5% to 14% throughout Europe and Asia. It appears to be a genetic polymorphism without much clinical impact in the absence of another genetic or environmental factor. Compound heterozygosity for C282Y/H63D is seen in 4% to 7% of persons with a hemochromatosis phenotype.
A second HFE mutation, replacement of histidine by aspartate at residue 63 of the HFE protein (H63D) is frequently found on the non-C282Y-containing chromosome of individuals with clinical hemochromatosis who are heterozygous for C282Y.6 H63D is an older mutation with a wider population distribution, having an allele frequency of 5% to 14% throughout Europe and Asia. It appears to be a genetic polymorphism without much clinical impact in the absence of another genetic or environmental factor. Compound heterozygosity for C282Y/H63D is seen in 4% to 7% of persons with a hemochromatosis phenotype.
![]() Seventeen additional polymorphisms in HFE have been described. Of these, only the S65C mutation appears to have clinical impact, and it may cause mild iron overload when the individual is compound heterozygous for C282Y or H63D.
Seventeen additional polymorphisms in HFE have been described. Of these, only the S65C mutation appears to have clinical impact, and it may cause mild iron overload when the individual is compound heterozygous for C282Y or H63D.
![]()
PATHOPHYSIOLOGY
Since iron excretion in the gut is fixed at 1 mg/day, normal iron balance must be maintained by meticulous control of iron absorption in the intestine and iron release from macrophages. These are modulated in response to body iron stores and the erythropoietic demand for iron.
Hepcidin: Key Regulator of Iron Homeostasis
Hepcidin, a liver-derived peptide hormone, is a key negative regulator of iron release into the plasma by intestinal enterocytes, macrophages, hepatocytes, and placental cells.8 It binds to and causes internalization and degradation of the cell surface iron exporter, ferroportin.9 Hepcidin excess decreases intestinal iron absorption and macrophage iron release, and causes anemia. Hepcidin deficiency promotes intestinal iron absorption and leads to tissue iron overload. Hepcidin gene expression is enhanced by iron overload and inflammation, and suppressed by anemia and hypoxia. Although hepcidin is ordinarily induced by dietary iron loading, its expression is inappropriately reduced in all forms of inherited hemochromatosis.10,11
Iron Overload Disorders and Hepcidin Deficiency
Hepcidin deficiency plays a central role in the pathogenesis of the inherited hemochromatosis disorders, including those due to mutations in the HFE gene, the hemojuvelin gene (HJV), the transferrin receptor 2 gene (TfR2), and hepcidin itself (HAMP) (Table 25.2). Hemojuvelin acts as a coreceptor in the bone morphogenetic protein (BMP) pathway, interacting with BMP ligands and BMP type I and II receptors to generate an active signaling complex.10 This complex activates a Smad receptor signaling cascade and translocation of a Smad complex to the nucleus, where it increases HFE transcription. HJV and HAMP mutations are critical components in the same common pathway; their negative effect on hepcidin expression is associated with severe iron loading in childhood, or juvenile hemochromatosis.
HFE Localization and Function
HFE is highly expressed in Kupffer cells of the liver and in tissue macrophages. Binding to β2 microglobulin (β2m) allows expression of HFE/β2m on the cell surface,12 where it forms a stable complex with transferrin receptor 1 (TfR1). The C282Y mutation prevents formation of a disulfide bond in HFE, disabling β2m binding, and preventing cell surface expression. Disruption of the HFE/β2m/TfR1 complex, and mutations in TfR2, are associated with adult-onset iron overload. HFE and TfR2 may regulate hepcidin expression by enhanced iron transport into the cell (endocytosis of diferric transferrin), by upstream regulation of hepcidin, or as weak coreceptors for BMP-SMAD signaling. According to this model, it might be possible to treat hemochromatosis by hepcidin replacement.
The most common form of secondary hemochromatosis is transfusional iron overload: 1 mL of red cells contains about 1 mg of iron. Inappropriate absorption of iron in the gut may also occur in association with ineffective erythropoiesis. In this case, the erythropoietic stimulus to decrease hepcidin levels overrides the effect of iron overload on increasing hepcidin expression.
Iron Homeostasis
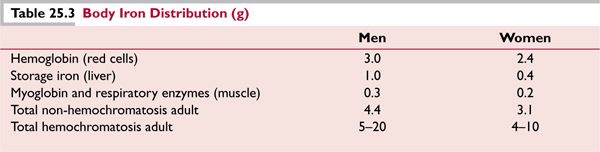
The distribution of body iron is shown in Table 25.3, with a comparison of iron stores in the normal state and in subjects with hemochromatosis.
![]() Excess iron and tissue injury. When the capacity for iron storage is exceeded, excess tissue iron causes cellular damage by catalyzing the formation of oxyradicals.13 Oxidative damage to lipids, proteins, carbohydrates, and DNA may lead to widespread impairment in cell function and integrity. In particular, lipid peroxidation may result in impaired membrane-dependent mitochondrial and lysosomal function. Oxidative injury to DNA, particularly in hepatocytes, may predispose to mutagenesis and cancer.
Excess iron and tissue injury. When the capacity for iron storage is exceeded, excess tissue iron causes cellular damage by catalyzing the formation of oxyradicals.13 Oxidative damage to lipids, proteins, carbohydrates, and DNA may lead to widespread impairment in cell function and integrity. In particular, lipid peroxidation may result in impaired membrane-dependent mitochondrial and lysosomal function. Oxidative injury to DNA, particularly in hepatocytes, may predispose to mutagenesis and cancer.
![]() Nontransferrin-bound iron (NTBI): This represents “free iron in serum.” NTBI enters cells freely, independent of receptor-mediated uptake. NTBI levels are low or undetectable at transferrin saturation (TS) below 40%, and increase linearly with TS levels above 40% to 50%. NTBI and its intracellular labile iron counterpart may be the direct mediators of oxidant stress. 13
Nontransferrin-bound iron (NTBI): This represents “free iron in serum.” NTBI enters cells freely, independent of receptor-mediated uptake. NTBI levels are low or undetectable at transferrin saturation (TS) below 40%, and increase linearly with TS levels above 40% to 50%. NTBI and its intracellular labile iron counterpart may be the direct mediators of oxidant stress. 13
![]()
CLINICAL FEATURES AND DIAGNOSIS OF HFE-HEMOCHROMATOSIS
Prior to the availability of biochemical and genetic screening tests, HFE-hemochromatosis was identified by damage to the liver, pancreas, heart, and joints, and diagnosed by demonstrating increased iron stores on liver biopsy. The “classic triad” of cirrhosis, diabetes, and skin pigmentation appeared in many publications and textbooks.1 Patients typically presented with the following symptoms:
![]() Severe liver disease due to hepatic fibrosis or cirrhosis
Severe liver disease due to hepatic fibrosis or cirrhosis
![]() Cardiac failure and refractory arrhythmias
Cardiac failure and refractory arrhythmias
![]() Polyendocrine failure: insulin-dependent diabetes and hypogonadotrophic hypogonadism
Polyendocrine failure: insulin-dependent diabetes and hypogonadotrophic hypogonadism
![]() Debilitating symmetric polyarthritis
Debilitating symmetric polyarthritis
![]() Grayish skin pigmentation
Grayish skin pigmentation
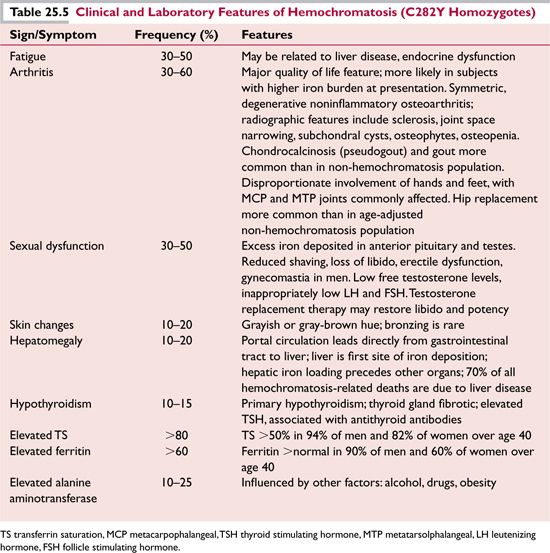
It is now recognized that this severe clinical phenotype is relatively rare and only develops in 1% to 4% of untreated C282Y homozygotes over their lifetime.4 Between 40% and 60% of C282Y homozygote males and 60% to 80% of homozygote females will remain asymptomatic or have minimal clinical manifestations throughout their lives; of the 40% to 50% who do develop symptoms that affect quality of life, arthritis, fatigue, and sexual dysfunction are the most common complaints (Table 25.4).14,15
New Diagnostic Definition
In the current era of molecular testing, recognizing that clinical penetrance can be highly variable, the diagnosis of hemochromatosis is established by the detection of two mutated HFE alleles. This definition does not require active symptoms or signs of illness or the presence of iron overload. Four stages of the disorder are recognized16:
1. Genetic predisposition with no other abnormality (age 0–20, 0–5 g tissue iron storage)
2. Iron overload without symptoms (age >20, >5 g iron storage)
3. Iron overload with early symptoms (age >30, >8 g iron storage)
4. Iron overload with organ damage (age >40, 10–20 g iron storage)
Common Clinical Presentation
The most common clinical presentation of hereditary hemochromatosis is with nonspecific symptoms, and therefore practitioners should have a low threshold for ordering serum TS and ferritin studies in patients with unexplained chronic fatigue, arthralgia, or arthritis, sexual dysfunction, hepatomegaly, or elevated liver function values (alanine aminotransferase [ALT]). Since symptoms are easily overlooked, the single most common event currently leading to a diagnosis of hemochromatosis is the incidental detection of an abnormal laboratory test result, either an elevated TS, serum ferritin, or ALT. In hemochromatosis subjects diagnosed with fatigue on presentation, screening laboratory tests to evaluate possible concomitant thyroid disease should be obtained.
Typical findings related to the most common clinical signs and symptoms of hemochromatosis are shown in Table 25.5. It is difficult to assign a frequency to these symptoms since there is a continuum of increasing frequency with increasing age, and with male versus female sex.16 Arthritis is the clinical feature with the greatest impact on quality of life.17 In contrast to significant cardiac abnormalities described in hemochromatosis patients who presented with very high iron prior to the advent of more frequent screening and the availability of a genetic test, heart disease now is generally absent or clinically insignificant in newly diagnosed, asymptomatic subjects.18
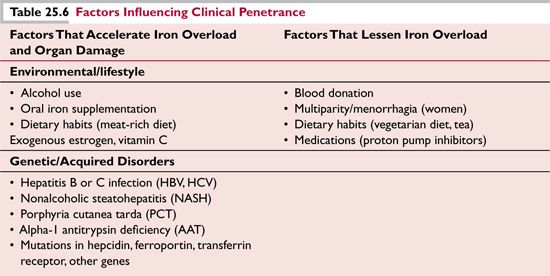
The considerable variability in clinical penetrance of C282Y homozygosity, both in rate of accumulation of iron stores and appearance of organ dysfunction, may be due to environmental, lifestyle, and genetic factors (Table 25.6).
![]()
LABORATORY TESTING
Once the clinical suspicion of hemochromatosis is raised, the diagnosis should be confirmed by laboratory testing, including those listed below:
![]() Serum iron, transferrin, and TS: Transferrin is the major iron transport protein in plasma. Several assay methods for TS exist: the most accurate is direct colorimetric analysis of serum iron (SI) combined with nephelometric assay of transferrin, wherein TS = molar concentration of iron divided by twice the molar concentration of transferrin. Less expensive but also less robust methods include chemical analyses of total serum iron binding capacity (TIBC) and unbound iron capacity (UIBC). Saturation of serum iron binding capacity is measured by dividing the serum iron by either TIBC (SI/TIBC) or by the sum of iron and UIBC [(SI)/(SI+
Serum iron, transferrin, and TS: Transferrin is the major iron transport protein in plasma. Several assay methods for TS exist: the most accurate is direct colorimetric analysis of serum iron (SI) combined with nephelometric assay of transferrin, wherein TS = molar concentration of iron divided by twice the molar concentration of transferrin. Less expensive but also less robust methods include chemical analyses of total serum iron binding capacity (TIBC) and unbound iron capacity (UIBC). Saturation of serum iron binding capacity is measured by dividing the serum iron by either TIBC (SI/TIBC) or by the sum of iron and UIBC [(SI)/(SI+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree