Hematopoietic stem cell transplantation (HSCT) offers potentially curative therapy for patients with myelodysplastic syndromes (MDS). However, as the majority of patients with MDS are in the seventh or eighth decade of life, conventional transplant regimens have been used only infrequently, and only with the development of reduced-intensity conditioning has transplantation been applied more broadly to older patients. Dependent upon disease status at the time of transplantation, 30% to 70% of patients can be expected to be cured of their disease and survive long term. However, posttransplant relapse and graft-versus-host disease (GVHD) remain problems and further investigations are needed.
The myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal bone marrow diseases characterized by ineffective production of normal mature blood cells and peripheral blood cytopenias. Generally required for the diagnosis are dysplastic changes in blood and marrow cells. In approximately one third of patients, MDS will eventually evolve to acute myeloid leukemia (AML). A detailed description of the disease characteristics, including the cellular and molecular biology of MDS, is provided elsewhere in this issue.
Infections caused by neutropenia and neutrophil dysfunction represent the leading cause of death in MDS. Life-threatening bleeding caused by thrombocytopenia is another complication directly attributable to marrow failure. The most frequent presentation, however, is anemia. Red blood cell transfusion dependence and the resulting iron overload may lead to additional organ complications, particularly in the heart, live,r and endocrine organs.
For most patients with MDS, chemotherapy alone is not a viable treatment option. Only about 40% of patients will achieve remissions, which are generally of short duration. For high-risk patients with MDS, 3-year survival rates after chemotherapy are in the range of only 5%.
Currently the only therapy with proven curative potential for MDS is hematopoietic stem cell transplantation (HSCT), with long-term survival rates between 25% and 70%. However, HSCT carries a risk of toxicity and potentially fatal complications, particularly in older patients. Given the frequently slow progression in low-risk MDS, the risk of treatment-related mortality (TRM) must be carefully weighed against the potential benefits of transplantation. Patient characteristics, timing of transplantation, and choice of conditioning regimen have to be considered. Thus, the questions are: transplantation for whom, when, and how? Should induction chemotherapy be given before HSCT? What should be the source of stem cells? Should the graft be T-cell depleted? How can one optimize the rate of engraftment and the graft-versus-tumor effect (GvT) while minimizing the incidence of graft-versus-host disease (GvHD) and TRM? The present article focuses on these questions.
General considerations
MDS originates in hematopoietic stem or precursor cells, and the goal of HSCT is to replace those cells and their progeny with cells from a healthy donor. Successful allogeneic HSCT requires that (1) healthy donor cells are able to establish themselves (engraft) in patients, and (2) the abnormal (malignant) cells responsible for the patients’ disease are eliminated or inactivated.
To allow donor cell engraftment it is generally necessary that patients are conditioned (ie, the immunologic barrier, which protects the body against intrusion by foreign organisms or cells, must be overcome). T-cell mediated host-versus-graft reactions seem to be the main cause of rejection, in addition to natural killer (NK)-cell effects.
Conditioning can include various immunosuppressive drugs, chemotherapeutic agents or irradiation, and new regimens are continuously being developed and tested. Regimens are often categorized as conventional/high-dose (myeloablative conditioning), reduced intensity (RIC), or low-dose/non-myeloablative conditioning. However, a review of the literature shows that there is a spectrum of regimens that basically form a continuum, and any categorization must remain artificial. Nevertheless, it is clear that the extent of toxicity correlates with conditioning intensity, and in general the probability of relapse is higher the lower the regimen intensity. This correlation is particularly true with transplantation for advanced disease. An important potential advantage of RIC is the ability of autologous marrow function to recover if the donor graft is rejected.
An undesired consequence of currently used conditioning regimens is that the defense against infectious organisms is also disrupted, and the fact that most conditioning regimens damage the anatomic tissue barriers (skin, mucosa) further enhances the risk of infections. This consequence was one rationale for the development of RIC regimens, which cause less tissue toxicity than conventional high-dose regimens, which in turn translates into lower acute non-relapse mortality (NRM). The development of these RIC regimens has also allowed to offer HSCT to older individuals who, until recently, were not considered transplant candidates, and TRM in various HSCT regimens has steadily declined in recent years.
A second purpose of the conditioning regimen is to ablate the abnormal cells responsible for patients’ disease. Although the conditioning regimens are generally effective in killing a large proportion of tumor cells, we rely on the immuno-therapeutic effect of donor cells for complete disease eradication. The lower the conditioning intensity, the more the patients’ cure will depend upon this GvT effect. In clinical studies, there is a strong correlation of GvT effect and GvHD, in particular chronic GvHD. Although recent research suggests that it may be possible to separate a GvT effect from the reactions that cause GvHD, clinically we have yet to show that such a separation is possible. Even if patients achieve remissions following HSCT, relapse remains a problem, especially in advanced MDS. The immunologic effects of HSCT, and consequently the risk of relapse and GvHD, depend upon the conditioning regimen, the nature of the graft (ie, degree of human leukocyte antigen [HLA]-match), stem-cell source (bone marrow, peripheral blood or umbilical cord blood), and GvHD prophylaxis, among others.
Only about 25% of patients will have HLA identical sibling donors (or matched related donors other than a sibling). However, large volunteer donor banks have been set up, and currently, an HLA-matched unrelated donor can be identified for about 50% to 60% of Caucasians; the proportion is lower for African Americans, and may be as low as 10% in some ethnic minorities. If a patient and sibling have inherited the same paternal and maternal chromosomes 6, they should be genotypically identical for HLA-A, -B, -C, -DR, and –DQ (ie, show a “10 out of 10” match). The only exception would be if a crossover has occurred. When we search for unrelated donors and typing by high resolution is available, we have the same objective of finding a “10 out of 10 match.” Lesser degrees of matching may be acceptable for some indications. With increasing degrees of mismatching, there is a higher probability of non-engraftment (particularly with HLA-C disparities) and a higher probability of GVHD. For patients without an HLA-matched related or unrelated donor, cord blood cells, or cells from a haploidentical-related donor may offer alternatives. Cord blood has the advantage of immaturity, allowing to transplant HLA-mismatched cells without a significant increase in GvHD incidence. A drawback is the limited number of cells available, often associated with a marked delay in engraftment. The use of two units of cord blood or in vitro expansion of the cord blood before infusion has partially overcome this limitation.
Initial results with haploidentical HSCT are encouraging. Graft failure presents only a minor problem, and GvHD rates have been surprisingly low, with relapse-free survival (RFS) and NRM comparing favorably with results from other HLA nonidentical transplants. It is clear, of course, that in addition to HLA antigens, so-called non-HLA and minor antigens (which are presented by HLA antigens) are involved in allogeneic interactions and GVHD (otherwise, no GVHD should be observed in HLA-genotypically identical sibling transplants). It is currently not routine to type donor and patient for those minor antigens.
In addition, research in recent years has shown that antigens typically expressed on NK cells, in particular killer-cell immunoglobulin-like receptor (KIR) antigens, are involved in donor/host interactions and may play an essential role in tumor cell elimination.
Risk assessment and scoring
Several MDS scoring systems have been developed and are used to identify patients who are likely to benefit from HSCT.
Multiparameter Prognostic Scoring Systems
The International Prognostic Scoring System (IPSS), used widely to assess patient prognosis, has also proven a reliable indicator of the probability of transplant success. The higher the IPSS score, the lower RFS. A major point of criticism is that the instrument’s predictions are based on data from time of diagnosis, although one recent study showed that if recalculated at the time of transplantation, the IPSS retained its predictive power. The more recently developed World Health Organization (WHO) Prognostic Scoring System (WPSS) includes WHO classification, karyotype, and transfusion dependence. In contrast to the IPSS it allows for real-time assessment of prognosis. A recent study validated its applicability to HSCT.
Another recent addition is the Simplified MDS Risk Score that includes poor performance status, older age, thrombocytopenia, anemia, increased marrow blasts, leukocytosis, chromosome 7 or complex (≥3) abnormalities, and earlier transfusions as adverse risk factors. It has been validated for secondary MDS and a cohort of de novo patients with MDS. Whether this system is truly simpler than others remains to be seen, but its value might lie in the fact that it incorporates performance status, which in turn might reflect comorbidities. A possible relevance in the context of HSCT remains to be determined.
Cytogenetics
Patients’ karyotype is the most powerful predictor of RFS after HSCT for MDS. This has led Armand and colleagues to reexamine the cytogenetic risk stratification of MDS in regards to the impact on transplant outcome. Their data, recently validated in a multicenter analysis, indicate that patients with MDS can be separated into two groups, good/intermediate versus poor-risk cytogenetics, with significantly differing outcome post-HSCT.
Flow Cytometry
Several studies have analyzed immunophenotypic aberrancies of MDS marrow cells and determined their prognostic relevance. Wells and colleagues developed a flow scoring system and showed a correlation of the severity of flow-cytometric aberrancies and the probability of relapse after HSCT. This was true even for patients with less than 5% marrow blasts at the time of HSCT. Presumably, differences in gene expression underlie the observed immunophenotypic abnormalities, and gene expression profiling has been shown to predict the risk for progression of MDS to AML.
Transfusion Dependence and Iron Overload
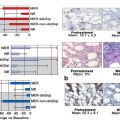
Several recent studies suggest that transfusion dependence (reflected in the WPSS) and iron overload have a negative impact on outcome after HSCT. The significance of elevated ferritin levels, however, is controversial, as ferritin may be elevated for various reasons, in particular inflammation. As such, a high ferritin level may reflect a different underlying disease process, rather than iron overload, although iron can contribute to inflammation. Liver iron content seems to be a more specific marker of iron overload, with noninvasive assessment procedures being developed. Treatment of iron overload may improve outcome following HSCT, although this is a matter of controversy.
Transfusion dependence is also linked to marrow fibrosis, the presence of which has long been recognized as being associated with accelerated disease progression in patients with MDS. Recent studies confirm those findings, and one study at least has shown a negative impact of fibrosis on post-HSCT outcome, particularly in patients with more advanced MDS.
Age and Comorbidity
Until the mid 1990s few patients over 55 years were offered allogeneic HSCT because of higher NRM in older patients. As only about 25% of patients with MDS are younger than 60 years, efforts have focused on reducing the elevated risk of NRM associated with older age by modifying conditioning regimens (as discussed earlier), supportive care, and complication management. Recent successfully transplanted cohorts included patients with median ages of 55 to 60 years, with some patients older than 70 years.
Much of the negative impact of age on prognosis appears to be caused by the frequency of comorbid conditions with older age. Sorror and colleagues developed a HCT-specific comorbidity index (HCT-CI) for risk assessment before transplantation, introducing objective laboratory and functional testing data, thereby modifying the Charlson Comorbidity Index. The instrument has been validated for patients with lymphoma and myeloma, and for a mixed patient sample. A multivariate analysis showed that the HCT-CI had greater predictive power for toxicities, NRM and overall survival after RIC HSCT than the Karnofksy Performance Score (KPS), but these findings were not confirmed in a Canadian study. However, the instruments measure two distinct patient characteristics, as evidenced by their weak correlation with each other. A combination of comorbidity and performance status assessment allows a more refined risk stratification for HSCT.
Risk assessment and scoring
Several MDS scoring systems have been developed and are used to identify patients who are likely to benefit from HSCT.
Multiparameter Prognostic Scoring Systems
The International Prognostic Scoring System (IPSS), used widely to assess patient prognosis, has also proven a reliable indicator of the probability of transplant success. The higher the IPSS score, the lower RFS. A major point of criticism is that the instrument’s predictions are based on data from time of diagnosis, although one recent study showed that if recalculated at the time of transplantation, the IPSS retained its predictive power. The more recently developed World Health Organization (WHO) Prognostic Scoring System (WPSS) includes WHO classification, karyotype, and transfusion dependence. In contrast to the IPSS it allows for real-time assessment of prognosis. A recent study validated its applicability to HSCT.
Another recent addition is the Simplified MDS Risk Score that includes poor performance status, older age, thrombocytopenia, anemia, increased marrow blasts, leukocytosis, chromosome 7 or complex (≥3) abnormalities, and earlier transfusions as adverse risk factors. It has been validated for secondary MDS and a cohort of de novo patients with MDS. Whether this system is truly simpler than others remains to be seen, but its value might lie in the fact that it incorporates performance status, which in turn might reflect comorbidities. A possible relevance in the context of HSCT remains to be determined.
Cytogenetics
Patients’ karyotype is the most powerful predictor of RFS after HSCT for MDS. This has led Armand and colleagues to reexamine the cytogenetic risk stratification of MDS in regards to the impact on transplant outcome. Their data, recently validated in a multicenter analysis, indicate that patients with MDS can be separated into two groups, good/intermediate versus poor-risk cytogenetics, with significantly differing outcome post-HSCT.
Flow Cytometry
Several studies have analyzed immunophenotypic aberrancies of MDS marrow cells and determined their prognostic relevance. Wells and colleagues developed a flow scoring system and showed a correlation of the severity of flow-cytometric aberrancies and the probability of relapse after HSCT. This was true even for patients with less than 5% marrow blasts at the time of HSCT. Presumably, differences in gene expression underlie the observed immunophenotypic abnormalities, and gene expression profiling has been shown to predict the risk for progression of MDS to AML.
Transfusion Dependence and Iron Overload
Several recent studies suggest that transfusion dependence (reflected in the WPSS) and iron overload have a negative impact on outcome after HSCT. The significance of elevated ferritin levels, however, is controversial, as ferritin may be elevated for various reasons, in particular inflammation. As such, a high ferritin level may reflect a different underlying disease process, rather than iron overload, although iron can contribute to inflammation. Liver iron content seems to be a more specific marker of iron overload, with noninvasive assessment procedures being developed. Treatment of iron overload may improve outcome following HSCT, although this is a matter of controversy.
Transfusion dependence is also linked to marrow fibrosis, the presence of which has long been recognized as being associated with accelerated disease progression in patients with MDS. Recent studies confirm those findings, and one study at least has shown a negative impact of fibrosis on post-HSCT outcome, particularly in patients with more advanced MDS.
Age and Comorbidity
Until the mid 1990s few patients over 55 years were offered allogeneic HSCT because of higher NRM in older patients. As only about 25% of patients with MDS are younger than 60 years, efforts have focused on reducing the elevated risk of NRM associated with older age by modifying conditioning regimens (as discussed earlier), supportive care, and complication management. Recent successfully transplanted cohorts included patients with median ages of 55 to 60 years, with some patients older than 70 years.
Much of the negative impact of age on prognosis appears to be caused by the frequency of comorbid conditions with older age. Sorror and colleagues developed a HCT-specific comorbidity index (HCT-CI) for risk assessment before transplantation, introducing objective laboratory and functional testing data, thereby modifying the Charlson Comorbidity Index. The instrument has been validated for patients with lymphoma and myeloma, and for a mixed patient sample. A multivariate analysis showed that the HCT-CI had greater predictive power for toxicities, NRM and overall survival after RIC HSCT than the Karnofksy Performance Score (KPS), but these findings were not confirmed in a Canadian study. However, the instruments measure two distinct patient characteristics, as evidenced by their weak correlation with each other. A combination of comorbidity and performance status assessment allows a more refined risk stratification for HSCT.
Timing of transplantation
Determining the optimal timing of HSCT for MDS has proven difficult since it involves weighing the benefit of early transplantation (reduced risk of disease progression and relapse) with high TRM (in some studies as high as 20%–25%), especially in patients in whom disease progression (even without HSCT) may be slow.
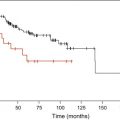
Generally, shorter disease duration before HSCT is linked to improved overall survival, decreased TRM and increased RFS. Lower blast count (based on French-American-British (FAB) classification) and younger age are also linked to more favorable outcome. Cutler and colleagues used a Markov model, involving a nontransplantation cohort and two patient cohorts receiving HLA-identical sibling transplants after high-dose conditioning, to determine the optimal timing of HSCT for MDS. They showed that patients with high or intermediate-2 risk by IPSS did benefit from early transplantation, whereas transplantation should be delayed for low-risk patients until evidence of disease progression. Intermediate-1 patients should probably be evaluated on a case by case basis. Although this analysis was restricted to patients transplanted from HLA-identical siblings, most transplant centers also apply this approach to patients transplanted from unrelated donors.
Al-Ali and colleagues found that outcome after allogeneic (and autologous) HSCT was best if transplantation was performed between 6 to 12 months after diagnosis, with higher overall survival and lower TRM than observed in patients transplanted later. They attributed the negative effect of later transplantation to frequent blood transfusion, longer duration of pancytopenias, and increased risk for progression during the waiting period for HSCT.
If transplantation has to be delayed, for example because of a lengthy search for an unrelated donor (median time 2–3 months ; median time from diagnosis to HSCT 12.9 months in the US ; median search duration 22 days for 549 patients in Germany ), bridging treatment with hypomethylating agents may delay progression to AML before HSCT. Such a strategy is especially relevant for patients who have MDS with IPSS intermediate-2 or high-risk disease, where the average time to AML progression may be short.
Pretransplant induction chemotherapy and the use of hypomethylating drugs
Studies linking elevated pretransplant blast counts to increased risk for relapse have led to investigations into the benefit of pretransplant induction chemotherapy for RFS after HSCT. Induction chemotherapy plays an important role in autologous HSCT, because this transplant modality requires that patients be in remission ; for allogeneic HSCT, the indication is less clear. Several retrospective studies suggest that patients who achieve remission after induction chemotherapy have a lower risk of relapse after HSCT than patients who do not respond to induction chemotherapy. It is still controversial whether this approach affects RFS.
It is conceivable that the effect of pretransplant chemotherapy consists in selecting treatment-sensitive patients. To address these questions, the European Group for Blood and Marrow Transplantation (EBMT) is currently conducting a prospective randomized phase III trial investigating the impact of pre-HSCT induction therapy on relapse rates after HSCT (EBMT Study code: Allo-MDS2x2).
Hypomethylating agents are emerging as an alternative to classic induction chemotherapy. In a recent retrospective study of therapy before allogeneic HSCT, overall survival, RFS, and cumulative incidence of relapse after 1 year were 47%, 41%, and 20%, respectively, for patients with MDS (and chronic myelogenous leukemia [CML]) receiving 5-azacytidine, compared with 60%, 51%, and 32% for non-5-azacytidine treated patients. Pretransplant administration of 5-azacytidine resulted in a trend to reduced risk of relapse. In 17 patients with MDS, the hypomethylating drug 2-deoxy-5-azacytidine (decitabine) did not negatively affect toxicity after HSCT, and disease downstaging may improve HSCT outcome. However, responses to both, 5-azacytidine and decitabine are often delayed; in cases requiring urgent action (eg, due to high risk for progression), induction chemotherapy may be preferable.
Conditioning regimens
Intensive research in recent years has been geared toward minimizing the toxicity while optimizing the efficacy of conditioning regimens. However, there is no one-size-fits-all conditioning regimen. Instead, conditioning should be tailored to diagnosis, disease stage, patient age, prior therapy, comorbidities, and the other components of HSCT, such as donor and stem cell source.
Although conventional conditioning is associated with a lower risk for relapse, its toxicity makes it unsuitable for many patients with comorbidities, and it is generally only offered to patients younger than 65 or 60 years with suitable related or unrelated donors, respectively. For MDS patients over 60 years, and those with comorbidities, RIC is a viable alternative. Although it is associated with a higher risk for relapse, this is possibly offset by lower TRM, thereby offering equivalent overall survival and RFS, although no prospective randomized study of comparable patients has been conducted so far. Even patients older than 70 years have been transplanted successfully using RIC. These results from retrospective studies should be interpreted with caution, because of likely bias in patient selection. Only prospective studies will allow a definite comparison.
Donor selection
The policy at most centers currently is to search for HLA-identical siblings. If no sibling is available, then an attempt is made to identify HLA-matched unrelated donors. Cord blood is being used as a third option. However, various centers have focused their research on the use of cord blood and might use this source of stem cells even instead of searching for a living unrelated donor. Further, efforts are underway to use haploidentical transplants more frequently since preliminary observations suggest a low rejection frequency and a surprisingly low incidence of GVHD. However, this approach must be considered investigational, and further data are required before firm recommendations can be made.
Stem cell source and manipulation
Stem cells obtained by bone marrow (BM) aspiration, umbilical cord blood cells (UCB), and granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood progenitor cells (PBPC) lead to different outcomes, because of different GvHD and GvT effects, the number and nature of cells transplanted, and the relative maturity or activation of cells.
A retrospective EBMT study, in agreement with data from FHCRC, showed that the use of G-CSF mobilized PBPC for HSCT from related donors in patients with MDS was associated with lower treatment failure rates (relapse and refractory disease) than the use of marrow in all MDS subgroups except refractory anemia. Data from a recent Markov decision model of choice between BM and PBPC grafts in HLA-matched related donor HSCT involving 1111 adult subjects conditioned with high-dose regimens and given unmanipulated grafts, showed significantly higher survival and better quality of life with PBPC, mainly because of lower risk of relapse, despite a higher incidence of GvHD. How PBSC compares with BM in unrelated HSCT is controversial, but a randomized prospective study in unrelated transplant recipients was just recently completed and results are pending (Blood and Marrow Transplant Clinical Trials Network protocol 0201).
A third option is, as discussed, the use of UCB transplantation. The introduction of two-unit transplants has helped to overcome restrictions associated with the low cell dose of UCB units, and in vitro expansion of UCB is emerging as a further option. Few studies are available on the use of UCB in MDS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree