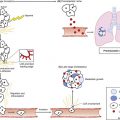
Qaiser Bashir and Richard Champlin • Hematopoietic cell transplantation (HCT) is a potentially curative treatment for a variety of hematopoietic, immune, metabolic, and malignant diseases. • Hematopoietic stem cells for transplantation can be collected from bone marrow, peripheral blood, or umbilical cord blood. • With the advent of unrelated donor, haploidentical, and cord blood transplantation, virtually every patient can now have a suitable donor. • Reduced-intensity conditioning regimens are associated with lower treatment-related mortality and allow for transplantation in elderly and patients with comorbidities who are not candidates for myeloablative HCT. • Allogeneic HCT is most frequently performed for patients with acute and chronic leukemias, myelodysplastic syndromes, immune deficiencies, metabolic disorders, and bone marrow failure states. • Autologous HCT is usually performed as part of the initial treatment for multiple myeloma, relapsed Hodgkin and non-Hodgkin lymphoma, and select solid tumors. • Common complications of HCT include regimen-related organ toxicity, graft rejection, graft-versus-host disease, infections, and secondary malignancies. Allogeneic HCT can be used if the patient’s bone marrow is involved by the malignancy or damaged by the disease or prior therapy. Allogeneic HCT carries the risk of immune complications including graft rejection (a situation in which the recipient rejects the donor cells) or graft-versus-host disease (GVHD) (a situation in which the immune cells from the donor react against recipient tissues). Allogeneic HCT may also confer a beneficial immune-mediated graft-versus-malignancy effect in which donor immune cells react against residual malignant cells.2 Compared with autologous transplants, allogeneic HCT is generally more effective in eradicating malignancies, but it has a greater risk of treatment-related mortality as a result of GVHD and infections. An international network of registries to provide an HLA-matched unrelated donor has been established. More than 18 million potential donors can be accessed worldwide. Initial studies of unrelated donor transplants reported a relatively high rate of GVHD and treatment-related mortality. Improved results have been achieved with donors and recipients matched for HLA-A, HLA-B, HLA-C, and DRB1 using high-resolution (allele level) typing,3 and recent results with matched unrelated donor transplants are similar to those achieved with matched sibling donors. After HCT, hematopoiesis and immunity are predominantly derived from donor-derived hematopoietic stem cells, although mature T cells in the graft contribute to initial immune recovery. Human hematopoietic stem cells and progenitor cells are contained in the CD34+ fraction of bone marrow cells, which constitutes approximately 1% of the bone marrow. Bone marrow was initially the source of hematopoietic cells for transplantation, collected by multiple aspirations with the patient under general anesthesia. Hematopoietic cells also circulate in the peripheral blood, and their frequency increases after treatment with growth factors and after chemotherapy and peripheral blood progenitor cells can be used for transplantation. Larger numbers of CD34+ cells can generally be collected as peripheral blood progenitor cells, and hematopoietic recovery is accelerated with peripheral blood progenitor cell transplants compared with bone marrow; as a result, almost all autologous HCTs utilize peripheral blood progenitor cells. Several studies have compared the outcome of allogeneic HCT using bone marrow or peripheral blood progenitor cells. These studies uniformly show more rapid time to engraftment with peripheral blood progenitor cells but an increased risk of chronic GVHD (cGVHD). Some investigators have suggested superior disease-free survival (DFS) in matched siblings using peripheral blood progenitor cells,4 but in most studies, overall survival (OS) was similar. Bone marrow has produced improved outcomes compared with peripheral blood progenitor cells in children and in patients with aplastic anemia. Umbilical cord blood is an alternative source of hematopoietic stem cells for transplantation. Cord blood units are obtained by collecting blood remaining in the umbilical cord and placenta after the delivery of an infant. Umbilical cord blood transplants are less likely to produce GVHD than are hematopoietic cells from adult donors, which allows successful transplants using unrelated units matched for four or five of the six HLA-A, HLA-B, and HLA-DR antigens.5 An international network of cord blood banks has been established, with collection of umbilical cord blood from volunteer unrelated donors after delivery of the newborn. Approximately 500,000 umbilical cord blood units are available worldwide. An umbilical cord blood unit provides a relatively low stem cell dose, which results in a slower pace of hematopoietic and immune recovery. Results are improved with higher cell doses and better HLA matching. Adequately matched umbilical cord blood units can be identified for most patients. Initial studies reported better results in children than in adults. A sufficient cell dose cannot be achieved with a single umbilical cord blood unit for most adult recipients; two umbilical cord blood units have been reported to improve outcomes and allow treatment of most adults. Centers focusing on this approach have reported results in children and adults similar to those obtained with matched bone marrow and peripheral blood progenitor cell transplants from unrelated adult donors. A recent registry analysis showed similar results with double umbilical cord blood transplants as with matched or one antigen mismatched unrelated donor peripheral blood progenitor cell transplants.6 Because the units are already collected and banked, umbilical cord blood transplants can generally be performed more rapidly than transplants from an unrelated adult donor. Haploidentical relatives are another potential donor source. Parents, children, and half of siblings are haploidentical, and thus these donors are readily available for most patients. Several centers have reported success with transplantation of T-cell–depleted peripheral blood progenitor cells with a low rate of GVHD.7 These transplants are associated with a relatively high rate of rejection, slow immune recovery, and a substantial risk of treatment-related mortality. An alternative approach uses unmodified haploidentical bone marrow transplantation with posttransplant treatment with cyclophosphamide, tacrolimus, and mycophenolate; this regimen produces a low rate of severe acute and chronic GVHD and treatment-related morbidity and mortality.8 Recent multicenter studies confirm that successful transplants can be performed in children and adult recipients from either unrelated cord blood or a haploidentical related donor9; overall, results have been similar with each approach and comparable with those reported with matched unrelated donors. Further studies are required to directly compare these strategies and to optimize results of HCT from every donor source. Use of these alternative donors provides an opportunity for treatment of nearly all patients with a clinical indication for HCT. A comparison of different sources of hematopoietic stem cells is presented in Table 30-1. Table 30-1 Comparison of Different Types of Allogeneic Transplantation *Grafts are occasionally cryopreserved. †For the purpose of this table, results with a related donor transplant are considered standard. This review focuses on HCT for treatment of cancer. HCT is performed for a wide array of hematologic malignancy and chemotherapy-sensitive solid tumors. Allogeneic HCT is associated with higher treatment-related mortality compared with autologous HCT, but it provides a graft that is free of disease, has a competent immune system, and confers the immune-mediated graft-versus-malignancy effect. Donor lymphocyte infusions (DLIs) can be given to augment this antitumor effect and may induce complete remission (CR) in selected patients with disease relapse after HCT.10 Because HCT is associated with significant morbidity and mortality, it is generally reserved for life-threatening diseases for which no other satisfactory treatment option exists. As new therapeutic options continue to evolve, so does the role of HCT. The indications for HCT vary by the disease type. Several organizations such as the European Blood and Marrow Transplantation11 and the National Comprehensive Cancer Network (www.nccn.org) have provided detailed treatment guidelines for specific diseases and clinical settings. The indications for HCT vary by disease type. Results are better in patients who have chemosensitive disease or are in remission. Outcomes of HCT are not favorable in patients with poor performance status and who have refractory or progressive disease. Evidence-based reviews have been published by the American Society for Blood and Marrow Transplantation regarding the role of blood and marrow transplantation in the treatment of selected disease.12 The numbers of patients receiving autologous and allogeneic transplantation for each major indication are shown in Figure 30-1. Allogeneic HCT is an optimal treatment for appropriately selected patients with acute myeloid leukemia (AML).13 Some centers have also successfully used autologous HCT for patients with AML who are experiencing their first or second remission. Recent advances have allowed risk stratification of patients with AML based on cytogenetic and molecular abnormalities, and this risk stratification helps guide therapeutic decisions.14 HCT is not recommended for patients in the “favorable” category. Large metaanalyses have shown that availability of an HLA-matched sibling did not result in superior DFS or OS with allogeneic HCT performed in the first period of remission for patients with favorable cytogenetics, t(8;21), inv16, or t(15;17). HCT prolongs DFS in patients with “intermediate-risk” and “high-risk” disease. Patients with diploid cytogenetics are considered intermediate risk, but recent studies have identified prognostically important subgroups based on molecular abnormalities. A large donor versus no-donor analysis of patients with cytogenetically normal AML showed that patients with the prognostically adverse FLT3-internal tandem duplication had improved DFS with allogeneic transplantation.15 Patients with nucleophosmin (NPM1) or CEBPA mutations without FLT3-internal tandem duplication had a better prognosis with chemotherapy, and there was a similar outcome with HCT compared with standard chemotherapy. HCT can also result in long-term DFS in approximately one third of patients with primary refractory AML. AML is most common in elderly persons. Reduced intensity conditioning HCT allows for successful transplantation in patients with AML who would otherwise be considered ineligible because of age or comorbidities. Disease relapse is an important concern after HCT. The prognosis of patients who relapse with AML after undergoing a transplant is poor. However, approximately 20% of patients with chemosensitive disease can achieve a durable remission with a second allogeneic transplant16 (see Chapter 98). Allogeneic HCT is an established indication for the treatment of myelodysplastic syndromes (MDSs).17 An international prognosis scoring system (IPSS) is used to classify patients with MDS into low, intermediate-1, intermediate-2, and high-risk categories based on bone marrow blast percentage, karyotype, and cytopenias.18 A modification of the IPSS system that incorporates a larger number of cytogenetic abnormalities, IPSS-R, was recently presented.19
Hematopoietic Stem Cell Transplantation
Introduction
Allogeneic HCT
Histocompatibility
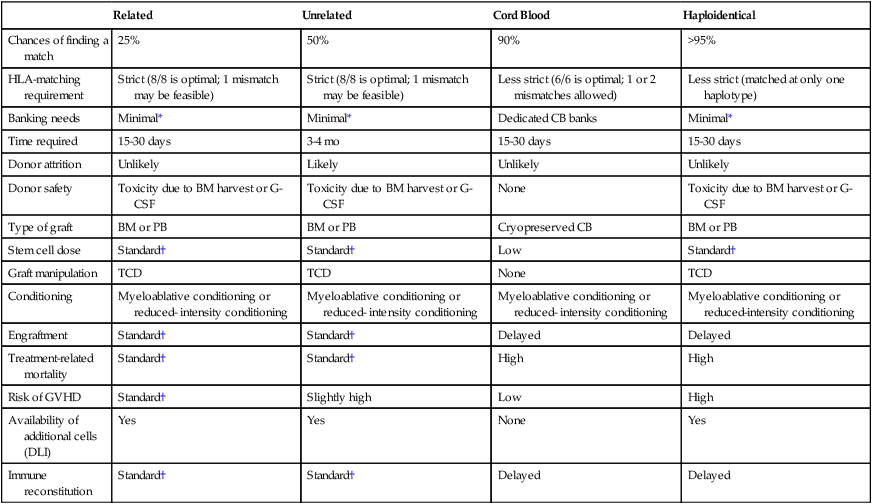
Cell Sources for HCT
Related
Unrelated
Cord Blood
Haploidentical
Chances of finding a match
25%
50%
90%
>95%
HLA-matching requirement
Strict (8/8 is optimal; 1 mismatch may be feasible)
Strict (8/8 is optimal; 1 mismatch may be feasible)
Less strict (6/6 is optimal; 1 or 2 mismatches allowed)
Less strict (matched at only one haplotype)
Banking needs
Minimal*
Minimal*
Dedicated CB banks
Minimal*
Time required
15-30 days
3-4 mo
15-30 days
15-30 days
Donor attrition
Unlikely
Likely
Unlikely
Unlikely
Donor safety
Toxicity due to BM harvest or G-CSF
Toxicity due to BM harvest or G-CSF
None
Toxicity due to BM harvest or G-CSF
Type of graft
BM or PB
BM or PB
Cryopreserved CB
BM or PB
Stem cell dose
Standard†
Standard†
Low
Standard†
Graft manipulation
TCD
TCD
None
TCD
Conditioning
Myeloablative conditioning or reduced- intensity conditioning
Myeloablative conditioning or reduced- intensity conditioning
Myeloablative conditioning or reduced- intensity conditioning
Myeloablative conditioning or reduced-intensity conditioning
Engraftment
Standard†
Standard†
Delayed
Delayed
Treatment-related mortality
Standard†
Standard†
High
High
Risk of GVHD
Standard†
Slightly high
Low
High
Availability of additional cells (DLI)
Yes
Yes
None
Yes
Immune reconstitution
Standard†
Standard†
Delayed
Delayed
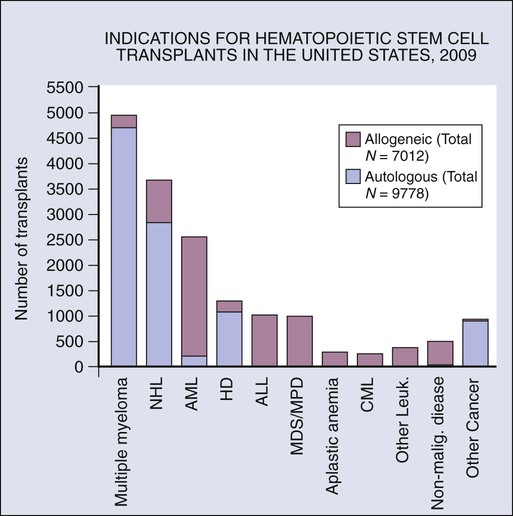
Disease Indications for HCT
Acute Myeloid Leukemia
Myelodysplastic Syndromes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Hematopoietic Stem Cell Transplantation