This review of hematology in Africa highlights areas of current practice and the immediate needs for development and clinical research. Acute hematological practice is dominated by anemia, sickle cell disease, and the need to provide a safe and rapidly available supply of blood. There is a growing need for specialist services for bleeding and coagulation, hematological malignancy, and palliative care. There are many areas of practice where straightforward measures could yield large gains in patient care. There is an urgent need for good clinical research to describe the epidemiology, natural history, and management of hematological diseases in Africa.
Key points
- •
Hematology practice in Africa is rapidly evolving to encompass the growing and changing demands in clinical and laboratory services and blood transfusion.
- •
Anemia is the most common hematological disorder, with iron deficiency the most common form, and is more prevalent in children (secondary to nutritional deficiency) and women (secondary to menorrhagia and pregnancy).
- •
The global burden of sickle cell disease (SCD) is highest in Africa, with significant birth prevalence, morbidity, and mortality. There are increasing efforts to introduce effective interventions.
- •
There is growing need for specialist services for bleeding and coagulation disorders, with increased incidence of thromboembolic disorders and an increase in the use of anticoagulation therapy.
- •
Hematological malignancies, such as leukemias and lymphomas, seem more prevalent and aggressive. The poor prognosis may be compounded by the late diagnosis and limited resources available to provide basic treatment protocols.
Introduction
The practice of hematology and blood transfusion (BT) is rapidly evolving in many countries in sub-Saharan Africa (SSA). Although SSA covers an enormous and diverse geographic area with a considerable range of economic and social circumstances, there are common themes that run through much of the medical and hematological practice of the continent. This review addresses the major topics of common concern, based on epidemiologic burden, existing programs and opportunities for addressing some of the major challenges in this field.
Many countries in SSA are going through similar socioeconomic changes that are influencing hematology practice. First, there is the epidemiologic transition that is occurring, with reduction in burden of infectious disease and increase in burden of noncommunicable diseases. This is accompanied by increased childhood survival and longer life expectancy. Hematological diseases are increasingly common and form a major part of everyday medicine across all specialties. Health systems (in both the public and private sectors) are growing stronger, leading to improvement in the diagnosis and management of hematological disorders. In many health facilities, hemoglobin concentration and blood counts from automated analyzers are available and are the most frequently requested laboratory tests. There has been strengthening of BT services with provision of safe blood components as an emergency, life-saving intervention or as part of planned interventions, for example, elective surgery.
The overwhelming focus of development of health services and training of health personnel has been to develop primary and secondary health services. Understandably, everyday hematological practice has fallen within the scope of primary health care workers. At secondary health care facilities, this has been provided by general medical staff in adult and pediatric departments. This provision of care has been necessary and sufficient to deal with common conditions, but it is now apparent that greater expertise is required, not only for the treatment of hematological diseases, but also for the wider development of hematological services. Over the past 2 decades, most of the hematological conditions requiring monitoring and follow-up have been occurring at tertiary-level health facilities. There is an increasing need, however, to improve the capacity to provide hematology services at primary-level and secondary-level health facilities. This is critical to decentralize and ensure that services are more accessible to a wider population and not limited to urban hospitals.
Furthermore, aspects of prevention and management of blood diseases require knowledge and improvement in practice in the community. As a consequence, many governments are investing in improving the capacity of the health care system to enable prompt recognition and referral of suspected cases from primary-level and secondary-level health facilities to tertiary-level hospitals as well as strengthening the development of specialist centers for diagnosis and treatment. Although there has previously been a limitation in human resources and infrastructure, many centers now have a critical mass of both clinical and laboratory hematological expertise and experience from national, regional and global partnerships.
Given this background of the current position of hematology in Africa and the extensive coverage of anemia, iron deficiency, infection, SCD, glucose 6-phosphate dehydrogenase deficiency, and BT in other articles in this issue, this review highlights some of the other areas of hematological diseases and also the scope for further development of hematological practice in SSA.
Introduction
The practice of hematology and blood transfusion (BT) is rapidly evolving in many countries in sub-Saharan Africa (SSA). Although SSA covers an enormous and diverse geographic area with a considerable range of economic and social circumstances, there are common themes that run through much of the medical and hematological practice of the continent. This review addresses the major topics of common concern, based on epidemiologic burden, existing programs and opportunities for addressing some of the major challenges in this field.
Many countries in SSA are going through similar socioeconomic changes that are influencing hematology practice. First, there is the epidemiologic transition that is occurring, with reduction in burden of infectious disease and increase in burden of noncommunicable diseases. This is accompanied by increased childhood survival and longer life expectancy. Hematological diseases are increasingly common and form a major part of everyday medicine across all specialties. Health systems (in both the public and private sectors) are growing stronger, leading to improvement in the diagnosis and management of hematological disorders. In many health facilities, hemoglobin concentration and blood counts from automated analyzers are available and are the most frequently requested laboratory tests. There has been strengthening of BT services with provision of safe blood components as an emergency, life-saving intervention or as part of planned interventions, for example, elective surgery.
The overwhelming focus of development of health services and training of health personnel has been to develop primary and secondary health services. Understandably, everyday hematological practice has fallen within the scope of primary health care workers. At secondary health care facilities, this has been provided by general medical staff in adult and pediatric departments. This provision of care has been necessary and sufficient to deal with common conditions, but it is now apparent that greater expertise is required, not only for the treatment of hematological diseases, but also for the wider development of hematological services. Over the past 2 decades, most of the hematological conditions requiring monitoring and follow-up have been occurring at tertiary-level health facilities. There is an increasing need, however, to improve the capacity to provide hematology services at primary-level and secondary-level health facilities. This is critical to decentralize and ensure that services are more accessible to a wider population and not limited to urban hospitals.
Furthermore, aspects of prevention and management of blood diseases require knowledge and improvement in practice in the community. As a consequence, many governments are investing in improving the capacity of the health care system to enable prompt recognition and referral of suspected cases from primary-level and secondary-level health facilities to tertiary-level hospitals as well as strengthening the development of specialist centers for diagnosis and treatment. Although there has previously been a limitation in human resources and infrastructure, many centers now have a critical mass of both clinical and laboratory hematological expertise and experience from national, regional and global partnerships.
Given this background of the current position of hematology in Africa and the extensive coverage of anemia, iron deficiency, infection, SCD, glucose 6-phosphate dehydrogenase deficiency, and BT in other articles in this issue, this review highlights some of the other areas of hematological diseases and also the scope for further development of hematological practice in SSA.
Red cell disorders
Anemia
Anemia is widespread throughout SSA and is frequently multifactorial in origin, but the major contributing factors are iron deficiency, malaria, anemia of chronic disease, and disorders of red cell, both hemoglobinopathies and enzymopathies. It has been estimated that more than 50% of preschool children, more than 60% of pregnant women and more than 40% of nonpregnant women are anemic.
The burden of disease caused by chronic anemia on individuals has been difficult to estimate. It is widely recognized that those with a hemoglobin of greater than 70 g/L can compensate for reduced levels of hemoglobin by increasing oxygen delivery through increasing red cell 2,3-diphosphoglycerate and increasing blood flow. Mild chronic anemia in children, however, typically due to iron deficiency and malaria, is associated with reduced growth and development, reduced neurocognitive function and learning and in many diseases with increased mortality and morbidity. The challenges are not only to improve treatment of severe acute anemia but also to understand and reduce the enormous burden of disease associated with chronic anemia.
Heart failure is recognized as a common complication of anemia that results in hospitalization and emergency BT. In many hospitals, a hemoglobin of less than 50 g/L is considered an indication for BT. Due to the rapidly life-threatening nature of severe anemia, however, the presence of clinical features of severe anemia and heart failure is often an indication of BT, even without the laboratory result of the hemoglobin. This practice has been critical to reduce mortality but has led to difficulty in understanding the causes of anemia. There are now an increasing number of studies, however, that are reporting on the etiology of anemia that have highlighted the contribution of bacterial infection to severe anemia and the contribution of anemia, in particular iron deficiency anemia (IDA), to heart failure.
Malaria
Falciparum malaria causes acute and chronic anemia. Severe malarial anemia due to Plasmodium falciparum carries low mortality when it appears in isolation but significantly contributes to increased mortality in those children where severe anemia (hemoglobin <50 g/L) is accompanied by cerebral malaria or respiratory distress.
The burden of anemia due to malaria is considerable and may account for 60% of episodes of anemia in young children. Where transmission is intense, younger children under 3 years of age present with isolated anemia. In areas of lower transmission, older children develop severe anemia and, for reasons not well understood, often accompanied by other syndromes of severe disease, such as cerebral malaria or respiratory distress. In many areas, older children with partial immunity to malaria may have chronic anemia with low parasitemia and marked dyserythropoiesis.
The complex etiology of malarial anemia has been described elsewhere (see David J. Roberts: Hematologic Changes Associated with Specific Infections in the Tropics , in this issue). The mainstays of therapy are now artemisinin derivatives for acute disease and oral artemisinin in combination with mefloquine (ASMQ), lumefantrine (Coartem), amodiaquine (ASAQ), piperaquine (Duo-Cotecxin), and pyronaridine (Pyramax) for oral therapy for less severely ill patients. The detailed chemotherapy of disease is beyond the scope of this article but is well described in a recent review from the World Health Organization (WHO).
The guidelines for blood transfusion in malaria are that transfusion should be given for those with hemoglobin less than 50 g/L or if there is evidence of respiratory distress at higher levels of hemoglobin concentration. There is little high-quality evidence, however, for more detailed guidance in complicated malaria, but transfusion is commonly given for anemic children (hemoglobin <100 g/L) with cerebral malaria with or without respiratory distress.
There is increasing evidence of the contribution of severe malaria and anemia to morbidity and mortality in SCD, despite the protection against malaria in the presence of sickle cell hemoglobin (HbS), particularly in individuals with heterozygous state of HbS. There is high mortality due to malaria in the homozygous state (HbSS). It is likely that this high mortality is a result of worsening hemolysis and anemia in individuals who already have a low steady state of hemoglobin.
Pregnancy
The maternal mortality rate is high in many African countries, making this one of the health-related millennium development goals. Tanzania reported that anemia and antepartum/postpartum hemorrhage account for up to 13% of maternal mortality. Maternal mortality rises rapidly when the hematocrit falls below 0.20, and low birthweight and perinatal and infant mortality rise if the hematocrit falls below 0.3. Reports from most BT services show that the highest demand for blood in terms of volume is from obstetrics and gynecology, whereas the highest demand in terms of numbers is from pediatrics. It is, therefore, critical to improve hematology and BT services to improve the diagnosis and management of the different hematological complications that result in morbidity and mortality during pregnancy.
Anemia is common in pregnancy and is associated with major morbidity and mortality ( Fig. 1 ). In malarial areas, pregnancy is associated with increased parasitemia and severity of acute malaria. The particular susceptibility factors to malaria include altered systemic immune responses and also specific local factors, including the ability of infected red cells to adhere to the syncytiotrophoblast in the placenta.
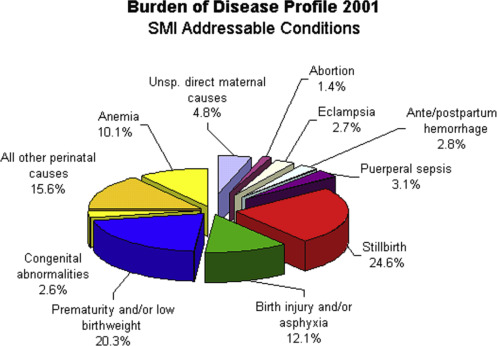
In nonimmune women, malaria infection is associated with severe complications, including anemia and cerebral malaria. Severe malarial anemia causes prematurity, intrauterine growth retardation, and increased maternal and infant mortality. In areas of high transmission, clinical malaria is increased with a peak instance in the second trimester. Multigravida women are less commonly symptomatic, reflecting the acquired immunity to specific malarial antigens that mediate adherence of infected cells to the placenta.
Diagnosis of malaria in pregnancy is difficult because the peripheral parasitemia may be low, even if placental vessels are heavily parasitized. Presumptive treatment of malaria is recommended for all severely anemic women exposed to malaria. Furthermore, most countries in Africa recommend intermittent treatment of malaria during pregnancy and in infants.
Iron Deficiency Anemia
Iron deficiency is the most common cause of anemia. This is more prevalent in pregnancy, particularly in multigravida women because iron stores are depleted by successive confinements. The treatment of IDA and supplementation of pregnant women to avoid iron deficiency is part of the recommended maternal and child health services. Given the links between pregnancy and malaria, presumptive treatment of malaria and/or intermittent preventive treatment of malaria is central to effective antenatal care.
Supplementation with folic acid (5 mg/d) and ferrous sulfate (200 mg/d) and anti-malarial prophylaxis form the backbone of treatment of malarial anemia in pregnancy. If maternal anemia is corrected at least 6 months before delivery, nearly normal neonatal outcomes ensue.
Hyperreactive Malarial Splenomegaly
Hyperreactive malarial splenomegaly (HMS) is characterized by chronic splenomegaly greater than 10 cm below the costal margin, elevated IgM (>2 SD above the mean), high titers of antimalarial antibodies, and lymphocytic infiltration of hepatic sinusoids. Treatment requires a curative course of chemotherapy for malaria and lifelong antimalarial prophylaxis. Splenectomy carries significant immediate perioperative and long-term infective risks and is not recommended.
Inherited Red Cell Disorders
The care and management of SCD dominate acute and chronic hematological diseases seen in Africa, but the distribution and severity of disease vary considerably. The disease distribution severity depends on the co-inheritance of ameliorating α + -thalassemia traits (in homozygous or heterozygous forms), the beta-globin haplotype, and other as-yet poorly characterized genetic and possibly environmental differences.
The level of fetal hemoglobin (HbF) has a substantial outcome on reducing the severity of disease. Polymorphisms at several loci are known to raise HbF, including genetic variants at 3 major genetic loci: Xmn1-HBG2, HMIP-2, and BCL11A. Recently, the influence of variants at these loci on the phenotype of SCD in Africa was shown in Tanzania, Cameroon, and Benin. The presence of the T allele at Xmn1-HBG2 led to a significant increase in hemoglobin ( P = 9.8 × 10[−3]); the BCL11A variant (rs11886868-‘C’) increases hemoglobin ( P = 2 × 10[−3]); and one of the HBS1L-MYB variants decreases white blood cell count values selectively ( P = 2.3 × 10[−4]). The distinct pattern of effects of each variant suggests that disease alleviation may occur, not only through increased HbF production, but also by indirect effects on blood cells through a variety of pathways.
The hemoglobin C allele is common in the Sahel region of West Africa and people with hemoglobin C disease (HbCC) and HbS hemoglobins present with chronic hemolytic anemia with a mild form of SCD with a marked predisposition to exacerbations during pregnancy ( Box 1 ).
- •
Homozygous HbCC causes mild hemolytic anemia.
- •
Steady state hemoglobin varies between 100 g/L and 120 g/L.
- •
Hemoglobin levels may fall to 90 g/L to 100 g/L in pregnancy.
- •
Folate deficiency may be severe.
- •
Iron deficiency is not uncommon.
- •
Peripheral blood shows target cells and microspheres.
- •
HbCC is readily diagnosed by mobility of a hemogolobin equivalent to hemoglobin A 2 on alkaline hemoglobin electrophoresis.
α + -Thalassemia trait in Africa is almost always due to the 1 gene deletion and even when inherited as a homozygous α + -thalassemia causes only mild asymptomatic microcytic anemia.
Sickle Cell Disease
The principal pathology and management of SCD is discussed elsewhere (see Thomas Williams: Sickle Cell Disease in Sub-Saharan Africa , in this issue). Rather than review the well-described clinical features, the focus in this article is on key factors that enhance the care of SCD in Africa.
First, newborn screening (NBS) for SCD would allow early prophylaxis and vaccination to prevent infection and prompt diagnosis and treatment of crises when children present to hospital. Reports from high-income countries have reported a 70% reduction in mortality in the 0- to 3-year age group that is thought to be a result of NBS and comprehensive care. Because most African countries do not have NBS for SCD as part of national health care program, this has been highlighted as a priority intervention to improve childhood survival in SCD.
Laboratory investigations for confirmation of diagnosis for SCD are not available in most hospitals in Africa. Therefore, efforts are being made to strengthen the diagnostic facilities in secondary and tertiary hospitals, particularly in areas with high prevalence of disease. NBS for SCD is being done using isoelectronic focusing or high-performance liquid chromatography to detect HbS. Due to the high prevalence of SCD in Africa and the absence of NBS for SCD in many countries, there is a strong justification for rapid diagnostic tests to detect hemoglobin variants by the bedside or at least at the point of care. There are increasing efforts being made to develop rapid point-of-care diagnostic tests and if protein-based or DNA-based assays cost less than $1, mass screening would be cost-effective.
The provision of comprehensive care for SCD has been found effective in reducing morbidity and mortality, and many African counties have been able to establish SCD services. Although the management of SCD requires a broad range of skilled specialist services, a minimum set of interventions can be introduced and provided at primary and secondary health care facilities. As such, the strategy in some African countries has been to develop SCD services as part of a package of noncommunicable disease services, for example, in Tanzania. This addresses the challenge that is faced by regional hospitals, and developing guidelines, training and staff to reach across the smaller towns and rural areas will require thoughtful and targeted training of clinical and laboratory staff. Such services will have to be delivered by nonspecialist medical or dedicated, trained nursing staff. Reaching out to spread SCD services across countries is a challenging, but achievable, goal.
Realizing these possibilities requires considerable raising of public awareness to promote such services. Public awareness of the nature of this inherited disease as a chance event and the treatable conditions associated with SCD will allow increased sympathy for patients and their families and allocation of more resources targeted at the diagnosis and care of patients with SCD. SCD societies exist in many countries and SCD awareness events are promoted across Africa. Support for patient societies can catalyze the allocation of resources and increase public awareness for SCD and should be an important aspect of hematological work.
Recent advances in the care of SCD include the use of hydroxyurea and long-term transfusion. Although there are barriers to implementing these strategies in Africa, there is increasing evidence that these interventions can be introduced. Most African countries should be able to provide penicillin prophylaxis for SCD in children under age 5.
There is increasing use of hydroxyurea for management of SCD in Africa. Extension of guidelines in North America or Europe suggests that chronic, if not lifelong, hydroxyurea would benefit patients with 1 life-threatening episode of complication of SCD or frequent hospitalizations with less severe disease. The safety and monitoring of patients on hydroxyurea have not been established in Africa. There are issues with compliance, cost and the increased incidence of invasive bacterial disease and malaria in African countries. Baseline data for such policy decisions, however, may soon be forthcoming, because several SCD centers have started to evaluate the use of hydroxyurea as well as beginning clinical trials. The cost of hydroxyurea at $0.66 per day is expensive when this is a high proportion of daily family income. Bulk purchases and international support could, however, overcome these problems, as has been proved for support for HIV, tuberculosis and malaria treatment by the Global Fund.
Chronic transfusion therapy for primary or secondary prevention of stroke is well established. It is possible that such therapy may be extended if the recent trials that demonstrated a reduction in silent infarcts by chronic transfusion therapy is confirmed. The implications of such transfusion therapy are considerable. These regimes require not only an adequate supply of blood but also sophisticated grouping, antibody screening, and identification and extended donor and recipient screening to reduce or manage alloimmunization after multiple transfusions. Developing transfusion services is a high priority because few centers at present could cope with such chronic transfusion regimes. Nevertheless, developing a program for the management of acute crises is essential.
The development of long-term hydroxyurea programs and transfusion therapy will require an extensive and robust evidence base from Africa and a wider health economic analysis.
With survival in SCD, the number of women with SCD requiring obstetric services is increasing. This is critical, because evidence shows that pregnancy in SCD is associated with poor fetal and maternal outcome. Further aspects of the obstetric care and treatment of the crises have been thoroughly reviewed, but specific guidance on key clinical scenarios have been given to highlight the salient parts in the management of sickle cell patients.
It is particularly evident when reviewing these guidelines that good clinical care depends on a wide range of services. Establishing, training and managing such teams at larger and at smaller hospital centers is critical.
Nutritional Anemia
Iron, folate, and vitamin B 12 deficiency are common in Africa and the diagnosis and treatment are often straightforward.
Iron Deficiency Anemia
The high prevalence of iron deficiency is due to several interacting factors. Diets are poor in iron, particularly in those in poverty who eat little animal protein; demand for iron is increased in infancy, in adolescence, and by menstruation and pregnancy, especially after multiple and/or closely spaced births. Iron loss may be increased by hookworm, schistosomiasis, Schistosoma haematobium , and Schistosoma mansoni in their respective geographic distributions. Finally, infection (in particular malaria) may contribute to raised hepcidin levels as part of a broad protective acute-phase response, which unfortunately also restricts iron absorption and mobilization of iron to developing erythroid cells. In many African countries, hookworm is considered the most common cause of IDA in children. Therefore, antihelminthic agents, such as mebendazole, are routinely prescribed for presumptive treatment.
The side effects of iron deficiency are not limited to anemia and fatigue but include impaired concentration, memory and attention. These neurocognitive effects are of great importance and one of many reasons for a focus on providing iron supplements to children on a large scale. In addition, there is increasing evidence that iron deficiency can cause heart failure, even in the absence of anemia.
The physiology of iron absorption and its diagnosis and management has been described elsewhere (see Pasricha, Drakesmith: Iron Deficiency Anemia – Problems in Diagnosis and Prevention at the Population Level , in this issue). Pivotal trials in Africa, however, have shown increased overall mortality in a community-wide iron supplementation trial in young children in Pemba, Tanzania. It seems that iron, particularly when given to non–iron-deficient children, remains unabsorbed and can be predisposed to increased bacterial infection, hospitalization, and mortality, possibly through alteration of the microbiome and promotion of pathogenic strains of commensal organisms. Moreover, iron deficiency may be protective from malaria infection, and the reticulocytosis in iron-deficient children receiving iron supplementation may increase malarial parasitemia.
The diagnosis of IDA in most African countries is challenging. Iron studies, such as serum iron, serum ferritin, transferrin saturation, and total iron-binding capacity, are not available in most facilities and when available, are expensive. Serum ferritin is the test that is the most available but its value is limited in individuals who have evidence of infection and inflammation because it is an acute-phase protein. As a consequence, the diagnosis of IDA is often made on the basis of a microcytic, hypochromic anemia.
Management of IDA is with oral formulations, although parenteral iron is used in the third trimester of pregnancy when rapid repletion of iron stores is required. Due to the high prevalence of iron and folate deficiency anemia, pregnant women are empirically prescribed supplementation.
After the risk of adverse outcomes after iron supplementation became clear, the WHO issued additional guidance that “caution (for iron and folate supplementation) should be exercised in settings where the prevalence of malaria and other infectious diseases is high.” Evidence-based guidance for the optimal rate and route of community-wide iron supplementation remains, however, unclear, in spite of widespread implementation of micronutrient powders that include iron.
Folate Deficiency
Active forms of folic acid form an essential component of 1-carbon metabolism that, among other things, is required for the conversion of uridine to thymidine for DNA synthesis and for the conversion of homocysteine to methionine.
Although many foods, in particular leaves ( folium is Latin for leaf), contain folic acid, it is easily destroyed by cooking, and body stores are not at all extensive, only sufficient for 3 to 6 weeks’ supply. Folate deficiency can, therefore, develop rapidly and be profound. Folate deficiency is commonly increased when cell turnover is enhanced, such as in hemolytic anemia and pregnancy and for much of normal development.
The diagnosis of folate deficiency is relatively easy, recognized by the features of macrocytic, megaloblastic anemia that may be accompanied by pancytopenia and mild hemolysis. There are challenges, however, with diagnosis in African countries where there may be mixed microcytic and macrocytic anemia due to iron deficiency and folate deficiency, respectively. In this instance, the red cell distribution width, which gives an indication of the heterogeneity of cell size, is a useful parameter. In the absence of red cell distribution width, which is available from most automated hematology analyzers, a blood film is used in the diagnosis, where the presence of hypersegmented neutrophils form a prominent part of the blood morphology in this condition. The measurement of serum or red cell folate is available in some African countries, but only in tertiary care hospitals, and is limited by the cost.
The management of folate deficiency is oral folic acid, given 5 mg per day. This is available and accessible in most African countries. Oral chemoprophylaxis for prevention of folate deficiency is recommended in neonates, in pregnant women and for chronic hemolytic anemia, such as SCD. In South Africa, co-ordination of cereal production has allowed straightforward fortification of maize flour. Such community-wide action may reduce neural tube defects in children, particularly because high levels of folic acid are required in the first trimester and, therefore, not amenable to improvement through attendance at antenatal clinics after the first trimester. Folate deficiency can also cause intrauterine growth retardation, prematurity and low birthweight.
Vitamin B 12 Deficiency
Vitamin B 12 deficiency is as common as folic acid deficiency. There is an overlap of the function of vitamin B 12 with folate in 1-carbon metabolism, thus the similarity of the clinical hematological syndromes. Vitamin B 12 is also needed, however, for myelination and is so severe that vitamin B 12 deficiency may present with subacute combined degeneration of the spinal cord.
Vitamin B 12 deficiency is not usually caused by dietary insufficiency but usually by autoimmune disease, leading to impairment of the absorption of vitamin B 12 by the intrinsic fact or chronic inflammation in the bowel. The diagnosis of vitamin B 12 deficiency often relies on clinical features, macrocytic hypochromic red cell indices and a megaloblastic blood film. As with diagnosis of folate deficiency, the laboratory tests for vitamin B 12 are not easily available due to access and costs. Management of vitamin B 12 deficiency is with parenteral injections. Although most hospitals do not have this available, it is readily available in private pharmacies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree