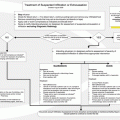
Adverse outcome predictor
Outcome point score
Age in years
1 per year
Male sex
+10
Cancer
+30
Heart failure
+10
Chronic lung disease
+10
Heart rate ≥110 bpm
+20
Systolic blood pressure <100 mm Hg
+30
Respiratory rate ≥30 breaths per minute
+20
Temperature <36 °C
+20
Altered mental status
+60
Arterial oxygen saturation <90 %
+20
Anticoagulation has been the mainstay of treatment of VTE in cancer patients with the goal of preventing thrombus propagation, which may lead to a fatal PE, pulmonary hypertension, and chronic lower extremity stasis and ulcers. Contraindications for anticoagulation are active bleeding, recent central nervous system bleeding, an intracranial or spinal lesion at high risk for bleeding (e.g., central nervous system metastases of melanoma, choriocarcinoma, thyroid cancer, renal cell cancer), and very recent major surgery. Other relative contraindications for anticoagulation include chronic bleeding, thrombocytopenia or platelet dysfunction, underlying coagulopathy, and a high risk of falls. Like with thrombolytic therapy, use of anticoagulation therapy should be justified according to the patient’s cancer status and overall therapeutic and palliative goals.
Anticoagulation therapy for acute VTE is performed in 2 phases: (1) initial treatment for 3–9 months according to the American College of Chest Physicians guidelines and (2) determination of the need for extended anticoagulation to reduce the risk of recurrent VTE in patients with ongoing identifiable risk factors or idiopathic/unprovoked VTE (Kearon et al. 2012). Initial anticoagulation therapy for acute VTE consists of administration of a parenteral anticoagulant to achieve an immediate antithrombotic effect, including subcutaneous injection of low-molecular-weight heparins (LMWHs) or IV infusion of weight-adjusted unfractionated heparin or oral rivaroxaban (Table 10.2). Agent selection should be based on the characteristics of the individual drug (half-life, mode of administration, reversibility, and cost) and the patient’s clinical situation (inpatient, outpatient, renal function, pending surgery, and pending thrombolytic therapy).
Table 10.2
Commonly used anticoagulants
Anticoagulant | Route and regimen | Situations for use |
|---|---|---|
Vitamin K antagonist | ||
Warfarin | PO | VTE, atrial fibrillation, and mechanical valve |
Factor Xa inhibitors | ||
Rivaroxaban | PO daily | VTE and atrial fibrillation |
Apixaban | PO daily | VTE and atrial fibrillation |
Fondaparinux | SC 5–10 mg daily | Adjust per CrCl and body weight |
Direct thrombin inhibitors | ||
Dabigatran etexilate | PO | Atrial fibrillation |
LMWHs | ||
Enoxaparin | SC 1 mg/kg every 12 h | Acute PE and symptomatic DVT |
SC 1.5 mg/kg daily | VTE, alternative to 1 mg/kg every 12 h | |
SC 1 mg/kg daily | CrCl < 30 mL/min | |
Dalteparin | SC 100 U/kg every 12 h | Acute PE and symptomatic DVT |
SC 200 U/kg daily | DVT treated on an outpatient basis | |
Unfractionated heparin | 80-U/kg IV bolus then 18 U/kg/h IV adjusted to twice the control aPTT | Acute VTE with high risk of bleeding or need for invasive procedures |
LMWHs are the preferred agents for initial therapy for acute VTE in most cancer patients. However, IV unfractionated heparin should be considered if rapid reversibility is needed, the patient will receive thrombolytic therapy, or the patient is morbidly obese or has significant renal insufficiency. LMWH given twice a day is preferred as the initial anticoagulation therapy in cancer patients with acute PE or severe symptomatic DVT. Dalteparin and enoxaparin should be used with caution in patients with creatinine clearance less than 30 mL a minute, whereas fondaparinux and tinzaparin use should be avoided in cancer patients with creatinine clearance less than 30 mL a minute. If feasible, anti-factor Xa activity should be monitored. LMWH dosing is adjusted to achieve optimal anticoagulation in these high-risk patients.
Maintenance anticoagulation therapy is required for cancer patients diagnosed with acute VTE: at least 3–6 months for those with DVT and at least 6–12 months for those with acute PE. However, use of anticoagulation therapy for an indefinite duration should be considered for patients with active cancer or other persistent risk factors. According to multiple national and international guidelines, LMWHs are the preferred agents for chronic anticoagulation for the first 6 months in cancer patients who have symptomatic VTE because anticoagulation with these agents is associated with superior outcomes in patients with solid tumors and symptomatic VTE (Lee et al. 2003; Kearon et al. 2012).
Oral vitamin K antagonists (e.g., warfarin ) remain reasonable treatment options for VTE when patients are unable to take LMWHs for a variety of reasons (adverse reactions, severe renal insufficiency, cost, or patient preference) or have completed 6 months of anticoagulation therapy with an LMWH. The initial dose of warfarin for most cancer patients with VTE should be no more than 5 mg daily. When warfarin use is initiated, parenteral anticoagulant administration for initial anticoagulation should be continued for at least 5 days and until the international normalized ratio (INR) has been within the therapeutic range for 24 h. The prothrombin time (PT) and INR should be checked at least twice a week in the first 2 weeks of treatment with warfarin until the INR is stabilized within the therapeutic range (2–3). The warfarin dose is then titrated based on the weekly or monthly INR.
IVC filters should only be used for treatment of acute VTE in cancer patients when anticoagulation is absolutely contraindicated or has failed. IVC filters also can be considered for patients with compromised cardiopulmonary function in whom another PE may be lethal. Other indications include prevention of PE during thrombolytic therapy or embolectomy for DVT. Permanent IVC filters should be used in patients with long-term contraindications for anticoagulation therapy, such as cerebral hemorrhage and high-risk brain metastases. In contrast, patients with temporary conditions requiring IVC filtration, such as surgery and trauma, should receive temporary retrievabl e IVC filters with strict follow-up for timely removal. Anticoagulation or concomitant anticoagulation therapy should be used as soon as the contraindications for this therapy resolve.
Thrombolytic therapy accelerates lysis of acute VTE and improves important physiologic parameters, such as right ventricular function and pulmonary perfusion. However, thrombolysis has not conclusively demonstrated a mortality benefit or been studied extensively in cancer patients with acute PE or VTE. Thrombolytic therapy is justified for hemodynamically unstable patients with massive PE or submassive PE along with evidence of moderate or severe right ventricular enlargement or dysfunction. Persistent hypotension owing to massive PE is the most widely accepted indication for thrombolytic therapy (Kearon et al. 2012).
Because thrombolytic therapy is associated with an increased risk of major hemorrhage, it should be considered only after PE is confirmed. If thrombolytic therapy is anticipated, such as with a patient who presents with a high clinical suspicion of PE and low blood pressure, any unnecessary invasive procedure should be avoided, and IV administration of unfractionated heparin but not LMWH should be considered for the initial anticoagulation while waiting for an imaging study to confirm the diagnosis. IV administration of 100 mg of alteplase over 2 h is the recommended thrombolytic regimen for PE in patients judged to be appropriate candidates for thrombolysis.
Thrombectomy and embolectomy can be performed via a catheter or surgically. Like thrombolytic therapy, embolectomy in cancer patients has yet to be studied in prospective clinical trials. Embolectomy should be considered when a cancer patient has massive PE or submassive PE with right ventricular dysfunction for whom thrombolytic therapy is justified but fails or is contraindicated. Similarly, thrombectomy is occasionally used in cancer patients with proximal occlusive DVT associated with significant swelling and symptoms for whom thrombolytic therapy fails or is contraindicated.
HVS
HVS is a clinical emergency consisting of increased blood viscosity resulting from increased levels of serum immunoglobulins (particularly IgM) as seen with diseases such as Waldenström macroglobulinemia and multiple myeloma. Less commonly, HVS can result from increased numbers of cellular blood components , such as red blood cells (RBCs) in polycythemia vera cases, platelets in thrombocytosis cases, and white blood cells (WBCs) in leukemia cases. Increased blood viscosity impedes capillary blood flow, leading to ischemia and organ dysfunction and resulting in a myriad of clinical manifestations: spontaneous mucous membrane bleeding, visual disturbances, and neurologic impairment. Other clinical complications include thrombosis, hypertension, heart failure, pulmonary congestion, and renal failure (Adams et al. 2009).
Serum viscosity (usually greater than 4 cP) is diagnostic in evaluating HVS when the increased viscosity is concomitant with characteristic symptoms, although serum viscosity measurements do not correlate well with symptoms or clinical findings. Other laboratory findings in HVS cases include rouleau formation on peripheral blood smears and a globulin gap of four or greater in multiple myeloma cases. Abnormal metabolic panels and electrolyte levels are common in patients with HVS.
The goal of treatment of HVS is to reduce plasma viscosity by removing excess cells or circulating complexes. Pending definitive therapy for underlying disease, supportive care should be initiated for the complications of HSV, including support for blood loss, central nervous disorders, cardiovascular effects, and metabolic imbalances. Plasmapheresis, the treatment of choice for acute severe HVS caused by paraproteinemia, rapidly reduces plasma viscosity by removing immunoglobulins, especially IgM, from the circulation, resulting in prompt alleviation of symptoms. This treatment is repeated daily until symptoms subside. If plasmapheresis is not available, vigorous IV hydration and withdrawal of 100–200 mL of blood may be performed to relieve symptoms in cases of acute HVS (Geraci et al. 1990). Of note is that plasmapheresis does not affect the underlying disease process; treatment of the underlying etiology should be initiated as soon as possible. Blood cell transfusions should be avoided until serum viscosity is reduced, as transfusion can increase the viscosity and worsen symptoms. If transfusion of packed RBCs (PRBCs) is necessary, it should be performed slowly and cautiously.
Hyperleukocytosis
Hyperleukocytosis is defined as a peripheral WBC count greater than 100 × 109/L. Leukostasis, or symptomatic hyperleukocytosis, is a medical emergency most commonly seen in patients with acute myeloid leukemia but is also associated with chronic myeloid leukemia in blast crisis, acute lymphoblastic leukemia, and chronic lymphocytic leukemia. Approximately 10–20 % of patients diagnosed with acute myeloid leukemia present with hyperleukocytosis. Large numbers of intravascular leukemic blasts increase blood viscosity and cause leukocyte aggregation and clumping in the microvasculature, resulting in end-organ damage and life-threatening complications. Leukostasis is diagnosed in a leukemia patient with a WBC count greater than 100 × 109/L and respiratory or neurologic manifestations.
The primary clinical symptoms of leukostasis are related to involvement of the lungs and central nervous system (Lester et al. 1985). Symptoms and signs of pulmonary leukostasis include dyspnea, tachypnea, and hypoxemia without hypercapnia. Pulmonary leukostasis may result in diffuse capillary leakage or adult respiratory distress syndrome or mimic acute PE with ventilation-perfusion mismatches (Kaminsky et al. 2000). Pulse oximetry is more accurate than arterial pO2 measurement in the assessment of oxygen saturation because WBCs consume oxygen in blood specimens obtained for analysis in test tubes. Neurologic signs and symptoms include vision-change headaches, dizziness, ataxia, stupor, confusion, and coma. Risk of intracranial hemorrhage is greatest after the leukocyte count decreases markedly, suggesting reperfusion injury as blood flow is restored to previously hypoxemic or ischemic capillary beds. Patients with leukostasis are often febrile owing to either inflammation associated with leukostasis or concurrent infection. Without treatment, the 1-week mortality rate is approximately 20–40 % (Bug et al. 2007). When both respiratory and neurologic status is compromised, the 1-week mortality rate reaches 90 % (Porcu et al. 1997).
The goal of treatment of leukostasis is to reduce the number of circulating leukocytes. Both chemotherapy and leukapheresis help rapidly reduce the number of circulating WBCs. However, only chemotherapy destroys leukemia cells in the bone marrow, which potentially improves survival. Systemic antileukemic therapy should be initiated early together with supportive care, such as adequate hydration, to reduce the risk of tumor lysis syndrome caused by rapid cell death. Alkalization of the urine and control of uric acid production using uricolytic agents (e.g., allopurinol, rasburicase) may be required to minimize the effects of urate nephropathy. Unnecessary blood cell transfusions should be avoided until the patient’s blast count decreases, as they can increase blood viscosity and worsen symptoms.
Leukapheresis likely is most helpful in leukemia patients with WBC counts greater than 100 × 109/L, high percentages of blasts, and neurologic or pulmonary leukostasis manifestations. Under appropriate conditions, leukapheresis can potentially decrease the WBC count by 30–60 %, with improvement in symptoms. Leukapheresis is not recommended for patients with acute promyelocytic leukemia (APL) because it is usually not effective and can potentially worsen the intrinsic coagulopathy associated with APL.
Thrombocytosis
Thrombocytosis is defined as a platelet count greater than 500 × 109/L and can be classified as either reactive or primary. Reactive thrombosis is the more common form, usually caused by chronic inflammatory or infectious disorders, surgery, hypersplenism, asplenia, hemorrhage, iron deficiency, malignancy, or medications such as eltrombopag, romiplostim, vincristine, all-trans retinoic acid (ATRA), cytokines, and growth factors. Most patients with reactive thrombocytosis do not have any symptoms or need any treatment.
Symptomatic primary thrombocytosis occurs in patients with essential thrombocythemia and polycythemia vera, whereas patients with chronic myeloid leukemia or myelofibrosis who have thrombocytosis are likely asymptomatic. Patients may have both thrombotic and hemorrhagic episodes, yet the correlation between the extent of thrombocytosis and the risk of thrombosis is poor (Michiels et al. 2006). Symptoms and complications of thrombocytosis result primarily from microvascular and macrovascular thrombotic events. Microvascular symptoms include acroparesthesia and digital ischemia, erythromelalgia, peripheral gangrene, and ischemic neurologic symptoms. Macrovascular thrombosis can occur in the legs, renal artery, and coronary, pelvic, splenic, and hepatic veins. Hemorrhagic events occur in up to 40 % of patients, with the gastrointestinal tract as the primary site of bleeding complications (Michiels et al. 2006).
Thrombocytosis is rarely an emergency and does not always require treatment. However, in patients with marked primary thrombocytosis and acute thrombosis, emergent plateletpheresis may be useful to rapidly decrease platelet counts to below 400 × 109/L (Regev et al. 1997). However, this provides only temporary control of the platelet count. Cytoreductive therapy (e.g., anagrelide, hydroxyurea), which is reserved for high-risk patients (age greater than 60 years, history of thrombosis, or platelet count greater than 1500 × 109/L), is usually necessary (Pescatore and Lindley 2000; Harrison et al. 2005). Patients should also take aspirin, although this should be done with caution, as platelet function defects are not uncommon.
Anemia
Almost all cancer patients experience anemia at one point during their disease. Most cases of anemia in cancer patients can have several superimposed factors (Table 10.3). Although the list of its mechanisms is daunting, causes of anemia can be simplistically categorized as increased blood loss as in hemorrhage or hemolysis or as decreased blood production.
Table 10.3
Causes of anemia in cancer patients
I. Blood loss |
A. Hemorrhage (see Acute Hemorrhage section and Table 10.8) B. Hemolysis (peripheral destruction) 1. Intrinsic/inherited a. RBC membrane: PNH, hereditary spherocytosis, hereditary elliptocytosis b. Hemoglobinopathies: sickle cell anemia, thalassemia c. Enzymes: G6PD deficiency, pyruvate kinase deficiency 2. Extrinsic/acquired a. Immune-mediated i. AIHA: warm and cold secondary to malignancies such as chronic lymphocytic leukemia, non-Hodgkin lymphoma, IgM gammopathy of unknown significance, solid tumors, and ovarian dermoid cysts ii. Rheumatologic: SLE, ulcerative colitis, common variable immune deficiency, autoimmune lymphoproliferative disease, postallogeneic SCT, and organ transplantation iii. Infections causing WAIHA: hepatitis C, A, and E and cytomegalovirus; infections causing CAIHA: mycoplasma and infectious mononucleosis iv. ABO-incompatibility transfusion reaction v. Drugs: sulfa, cephalosporins, quinidine, thiazides, NSAIDS, MTX, 5-FU, rifampin, ribavirin, sulfonylureas, and interferon-α b. Mechanical hemolysis: hemolytic uremic syndrome, TTP, DIC, and hypersplenism c. Infections and chemicals: malaria and hypotonic fluid |
II. Decreased production of RBCs |
A. Acute 1. Aplastic anemia 2. Acute leukemia 3. Overimposed infection: human parvovirus B19 4. Chemotherapy and radiation therapy 5. Myelophthisic anemia: primary myelofibrosis and metastatic solid tumors (breast, lung, and prostate cancer) B. Chronic 1. Nutritional deficiency: iron, folate, and vitamin B12 deficiency (owing to increased RBC turnover) 2. Ineffective erythropoiesis, MTX, and poor intake 3. Resulting from underlying disease: hypoendocrine state (thyroid, adrenal, pituitary), uremia, chronic inflammation, liver disease |
The spectrum of anemia at presentation is very broad, ranging from asymptomatic to severe depending on factors such as the underlying disease, patient’s age and cardiopulmonary reserves, and anemia severity and acuteness. Common symptoms of anemia result from decreased oxygen delivery to tissues, including fatigue, weakness, headaches, dyspnea, palpitations, and dizziness. Patients may have worsening of symptoms of their pre-existing underlying diseases, such as coronary artery disease. Physical findings of anemia include pallor, tachycardia, and systolic ejection murmur. Syncope and hypotension can occur when acute anemia results from massive blood loss resulting in hypovolemia.
Evaluation of anemia begins with a careful history, physical examination with particular attention to symptoms and signs of acute bleeding, and laboratory tests (Table 10.4). A complete blood cell count, particularly mean corpuscular volume measurement, reticulocyte count, the reticulocyte production index or reticulocyte index (RI), and a peripheral blood smear provide the initial guidance in the differential diagnosis of anemia. The RI is reticulocyte count × (measured hematocrit/normal hematocrit)/maturation correction. An RI greater than 2 % suggests a healthy response of bone marrow to acute blood loss or hemolysis, whereas an RI less than 2 % suggests inadequate production of RBCs. A peripheral blood smear can identify morphologic abnormalities and cell inclusions (Table 10.5). Further evaluation will depend on suspected causes of anemia, including LDH, indirect bilirubin, and haptoglobin measurement; urinalysis for hemoglobinuria; a direct antiglobulin test (DAT; or Coombs test); and serum iron, ferritin, total iron-binding capacity, vitamin B12, folate, RBC folate, creatinine, and thyroid-stimulating hormone measurement.
Table 10.4
Evaluation of anemia
RBC indices |
• Normal mean corpuscular volume and MCHC (normochromic, normocytic anemia) – Anemia of chronic disease – Hemolytic anemia – Anemia of acute hemorrhage – Aplastic anemia • Low mean corpuscular volume and low MCHC (hypochromic, microcytic anemia) – Iron deficiency anemia – Thalassemias • High mean corpuscular volume and normal MCHC (normochromic, macrocytic anemia) – Vitamin B12 deficiency – Folate deficiency |
RI |
• Calculation RI = (reticulocyte count × measured HCT/normal HCT)/maturation correction • Maturation correction For HCT: 36 % to 45 % → 1.0, 26–35 % → 1.5, 16–25 % → 2.0, and ≤15 % → 2.5 • Normal RI range is 1–3 %, with a mean of 2 %; indication of healthy bone marrow responding to acute blood loss |
Suspected hemolytic anemia |
• LDH measurement • Haptoglobin measurement • Indirect bilirubin measurement • DAT (Coombs test) • Urinalysis for hemoglobinuria |
Suspected chronic conditions for anemia |
• Ferritin measurement • Total iron-binding capacity measurement • Serum iron measurement • Vitamin B12 measurement • Folate and RBC folate measurement • Creatinine measurement • Thyroid-stimulating hormone measurement |
Anticipation of treatment (also see Table 10.8) |
• Type and cross for PRBCs • Platelets (pooled, apheresis, leukoreduction, irradiation, cytomegalovirus-negative, human leukocyte antigen matching, IgA-removed) • FFP • 4F-PCC • Cryoprecipitates • WBC transfusion |
Table 10.5
Evaluation of anemia: blood smears
I. Bone marrow infiltration (myelophthisis) |
A. Leukoerythroblastic changes 1. Nucleated RBCs 2. Immature WBCs (e.g., myelocytes, metamyelocytes, occasionally myeloblasts) B. Teardrop forms C. Giant platelets |
II. Hemolysis |
A. Spherocytes B. Polychromasia |
III. Mechanical hemolysis (DIC, TTP, prosthetic heart valve) |
Schistocytes (RBC fragments) |
IV. Iron deficiency anemia |
A. Hypochromia (pale RBCs) B. Microcytosis (small RBCs) C. Poikilocytosis (variation in shape) D. Anisocytosis (variation in size) E. Pencil-shaped cells |
V. Vitamin B12/folate deficiency |
A. Macro-ovalocytes (mean corpuscular volume > 115 fl) B. Anisocytosis C. Poikilocytosis D. Hypersegmented neutrophils |
VI. Glucose-6-phosphate dehydrogenase deficiency |
A. Heinz bodies (denatured hemoglobin) B. Bite and blister cells (from removal of Heinz bodies from the spleen) |
VII. Renal failure (burr cells) |
VIII. Liver disease (acanthocytes and target cells) |
Management of anemia varies depending on the underlying cause, severity of symptoms, functional status, co-morbidities, and hemoglobin level. Typically, transfusion of PRBCs is required for symptomatic severe anemia or acute anemia with active bleeding in a patient with a hemoglobin level of 7–9 g/dL, although this threshold is controversial. At The University of Texas MD Anderson Cancer Center, a hemoglobin level of at least 9 g/dL is the goal for patients with leukemia. In patients with solid cancers without stem cell problems, recombinant human erythropoietin is administered to reduce the number of transfused PRBCs. The benefits of transfusion should be carefully weighed against its risks, such as transfusion reactions, anaphylaxis, volume overload, infection, and iron overload in cases with chronic transfusion.
Hemolytic Anemia
Hemolytic anemia, or peripheral destruction of RBCs, may be either inherited (intrinsic) or acquired (extrinsic). Inherited conditions lead to defects in (1) the RBC membrane, such as hereditary spherocytosis, hereditary elliptocytosis, and paroxysmal nocturnal hemoglobinuria (PNH); (2) hemoglobin, such as sickle cell anemia and thalassemia; and (3) enzymes, such as glucose-6-phosphate dehydrogenase deficiency and pyruvate kinase deficiency. Acquired and extrinsic causes of hemolytic anemia are classified as follows: (1) immune-mediated, as in (a) autoimmune (lupus, chronic lymphocytic leukemia, non-Hodgkin lymphoma, stem cell transplantation) and (b) drug-induced (acetaminophen, nonsteroidal anti-inflammatory drugs, sulfa drugs, rifampin, cephalosporins, ribavirin, quinidine, thiazide, methotrexate, 5-fluorouracil) anemia; (2) infectious (viral hepatitis, cytomegalovirus, mycoplasma, Epstein-Barr virus, human immunodeficiency virus); and (3) mechanical, such as (a) prosthetic heart valves, infection with malaria, spider and snake venom, and hemodialysis; and (b) microangiopathic hemolytic anemia and its causes of thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome, disseminated intravascular coagulation (DIC), pre-eclampsia, and eclampsia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree