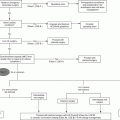
Definition of bleeding:
Minor bleeding – any clinically overt sign of hemorrhage (including imaging) that is associated with a <5 g/dl decrease in the hemoglobin concentration or <15 % decrease in the hematocrit felt by the clinician to be related to anticoagulation
Major bleeding – intracranial hemorrhage or a ≥5 g/dl decrease in the hemoglobin concentration or a ≥15 % absolute decrease in the hematocrit resulting in hemodynamic compromise or compression of a vital structure and felt by the clinician to be related to anticoagulation
Antiplatelet agents
Aspirin
Minor – desmopressin 0.3 mcg/kg × 1
Major – platelet transfusion
Clopidogrel (Plavix®)
Minor – desmopressin 0.3 mcg/kg × 1
Major – platelet transfusion, consider two units if life- or brain-threatening bleeding
Prasugrel (Effient®)
Minor – desmopressin 0.3 mcg/kg × 1
Major – platelet transfusion, consider two units if life- or brain-threatening bleeding
Ticagrelor (Brilinta®)
Minor – desmopressin 0.3 mcg/kg × 1
Major – platelet transfusion, consider two units if life- or brain-threatening bleeding
Sustained release aspirin/dipyridamole (Aggrenox®)
Minor – desmopressin 0.3 mcg/kg × 1
Major – platelet transfusion
Abciximab (ReoPro®)
Major – platelet transfusion
Eptifibatide (Integrilin®)
Minor – desmopressin 0.3 mcg/kg × 1
Major bleeding reversal: platelet transfusions plus infusion of 10 units of cryoprecipitate
Tirofiban (Aggrastat®)
Minor – desmopressin 0.3 mcg/kg × 1
Major bleeding reversal: platelet transfusions plus infusion of 10 units of cryoprecipitate
Heparin and heparin-like agents
Standard heparin
Time since last heparin dose
Dose of protamine
<30 min
1 unit/100 units of heparin
30–60 min
0.5–0.75 units/100 units of heparin
60–120 min
0.375–0.5 units/100 units of heparin
>120 min
0.25–0.375 units/100 units of heparin
Infusion rate should not exceed 5 mg/min. Maximum dose is 50 mg per dose
Low molecular weight heparin
Reversal of bleeding: protamine (works just as well with LMWH as heparin) – if within 4 h of dose, give 1 mg of protamine for each 1 mg of enoxaparin or 100 units of dalteparin and tinzaparin. Repeat one-half dose of protamine in 4 h. If 4–8 h after dose, give 0.5 mg for each 1 mg of enoxaparin or 100 units of dalteparin and tinzaparin.
Fondaparinux (Arixtra®)
Major bleeding reversal – protamine ineffective, rVIIa (90 mcg/kg) may be of use
Dabigatran (Pradaxa®)
Reverse if patient shows signs of bleeding and had an elevated aPTT >40 s
1. Profilnine (factor IX complex) 4,000 units (50 units/kg for patients under 80 kg) plus 1 mg of rfVIIa
Rivaroxaban (Xarelto®)
Reverse if patient shows signs of bleeding and has an INR >1.5
1. Profilnine (factor IX complex) 4,000 units (50 units/kg for patients under 80 kg) plus 1 mg of rfVIIa
Thrombolytic therapy
Reversal: immediate infusions of equivalent of 6–8 units of platelets (or one platelet pheresis product), 2 units of plasma, and 10 units of cryoprecipitate. No value in infusing antifibrinolytic agents
Medication of an Aged Population That Affects the Hematologic System
While not a natural change with aging, any discussion of the changes in the hematologic system with age, especially related to trauma, would be remiss not to mention the common medications that are prescribed. Antiplatelet therapy with aspirin or platelet ADP antagonists (clopidogrel, prasugrel, or ticagrelor) is common in the older trauma population. It is estimated that 50 million Americans are on aspirin therapy [36], and 29 million are prescribed clopidogrel or similar agents [37]. Pre-injury treatment with these drugs has been shown to increase trauma mortality especially with head injury [38, 39]. Aspirin inhibits platelet cyclooxygenase-1, which then blocks thromboxane A2 generation and subsequently inhibits platelet function. Clopidogrel-like drugs work by inhibiting the ADP-dependent mechanism of platelet aggregation. Both aspirin and ADP antagonists are reversed by platelet transfusion. Laboratory studies indicate that while one pheresis unit (6 random units) can reverse the aspirin defect, 2 pheresis units may be needed for ADP antagonists [40].
Desmopressin also has been shown to improve platelet function in the presence of these antiplatelet agents. Ranucci showed improved platelet function from 48 to 71 % by thromboelastography (TEG) with desmopressin therapy [41]. However, more clinical trials need to be performed before desmopressin should be included in antiplatelet reversal protocols.
There is increasing interest in using point-of-care assays such as VerifyNow to guide reversal therapy for antiplatelet agents. Bansal showed that many patients allegedly on antiplatelet agents had normal platelet function, and Bachelani showed that these assays may help guide platelet therapy [42, 43]. More widespread use of this type of laboratory testing may help avoid unnecessary transfusion and result in more efficient reversal of antiplatelet agents.
One of the leading medical indications for anticoagulation in the older population is atrial fibrillation. The prevalence of atrial fibrillation is steadily increasing [44], and it is estimated that by 2030, four million people in the USA will suffer from it [45]. While studies have shown that over a lifetime the benefit of reduced stroke rate with anticoagulation for atrial fibrillation does outweigh the bleeding risk, in an acutely injured patient, anticoagulation can be life-threatening. Warfarin acts by antagonizing vitamin K and thus inhibiting the vitamin K-dependent coagulation factors II, VII, IX, and X [46]. Pre-trauma treatment with warfarin has been associated with worse outcomes especially in patients with intracranial injury [47]. Rapid reversal protocols utilizing fresh frozen plasma (FFP) have been shown to reduce further bleeding and improve outcomes [39]. While traditional reversal relies on the transfusion of FFP and the administration of vitamin K, the process can be lengthy due to the time needed to thaw the plasma and infuse the product. Another issue is that substantial amounts of FFP are required for adequate reversal (15–20 ml/kg). The large volume of blood products may be detrimental to patients at risk for congestive heart failure.
Prothrombin complex concentrates (PCCs) contain variable concentrations of factors II, VII, IX, and X. Currently, due to the concern of thrombosis, only 3-factor concentrates containing minimal factor VII are currently available in the USA, but 4-factor concentrates are approved in Europe and Canada. PCCs are FDA approved for use in patients with hemophilia B. PCCs have several advantages over fresh frozen plasma. They are readily available and do not require infusion of large volumes of fluid. They are virally inactivated and have a reduced risk of causing transfusion-related acute lung injury. Reported uses include reversal of vitamin K antagonists, surgical procedures in hemophiliacs, cardiopulmonary bypass, and massive bleeding. PCCs have been associated with more rapid normalization of the INR compared to FFP, decreased incidence of hematoma enlargement, and improved neurologic outcomes in patients with anticoagulant-associated intracerebral hemorrhage [48] (Box 7.1).
Our current approach to reversing warfarin is to give PCC along with 1 mg of recombinant factor VIIa as a source of factor VII plus vitamin K for long-term warfarin reversal (Box 7.2). In the future when 4-factor PCCs are approved, these will become the product of choice for reversing warfarin [49].
Newer anticoagulant agents including dabigatran (direct thrombin inhibitor), rivaroxaban (factor Xa inhibitor), and apixaban (factor Xa inhibitor) are now being used in the elderly population. These drugs have advantages over warfarin in that they do not require lab monitoring, and they have much less drug interactions and no food interactions. All these agents have been shown to be equally or more effective than warfarin with respect to stroke prevention and safer with respect to intracranial hemorrhage complications. They are all options for stroke prevention in atrial fibrillation [50]. In addition, both dabigatran and rivaroxaban have been shown to be equal to warfarin in the therapy of venous thrombosis [51]. At this time there are no direct reversal agents for these new drugs. However, animal studies have suggested that PCC may be effective at halting bleeding, and at our institution, we use PCC + rVIIa combination for bleeding with these new agents [52, 53].
Box 7.2 OHSU Warfarin Reversal Protocol
For patients who present with warfarin and demonstrated ICH with an INR >1.45 |
1. Profilnine 4,000 units |
(a) 50 units/kg for patients under 80 kg |
2. 1 mg of rfVIIa |
3. 10 mg vitamin K slow IV infusion |
4. INR check after infusions |
(a) If still >1.5 repeat with 2,000 units dosing |
Conclusion
Considering the complexity of the hematologic system, its function is very well preserved as an individual ages, but the trauma physician needs to be aware of the slight changes that impact the older injured patient. An older patient will likely present with a lower baseline hemoglobin. It will be more difficult for the patient to recover from any hemorrhage secondary to the bone marrow and hematopoietic stem cell changes that are part of normal aging. After the initial injury, the older patient will return to their slightly hypercoagulable state which is important to consider in their recovery. Finally, with the prescribing practices in the USA, the older patient may be on certain medications that affect the hematologic system and can have negative impacts on their post-injury recovery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree