, Amin Alousi1, Joyce Neumann1 and Richard Champlin1
(1)
Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
This chapter will review the late effects of treatment impacting hematopoietic stem cell transplantation (HSCT) survivors. In general, HSCT patients receive high doses of chemotherapy with or without radiation therapy to eradicate their malignancy, together with an infusion of their own (autologous) or another person’s (allogeneic) stem cells to restore hematopoiesis (the blood and immune system). Allogeneic cells may be from bone marrow, peripheral blood, or umbilical cord blood obtained from a related or unrelated donor. Patients experience the toxic effects of the cytotoxic treatment and are at high risk for infections owing to posttransplant immune deficiency. Late effects for HSCT survivors are commonly compounded by the toxic effects of their previous cancer treatment. This chapter will cover physiologic and psychological aspects of survivorship for HSCT patients, as well as graft-versus-host disease, a common and frequently chronic condition that occurs after allogeneic HSCT.
Unique Needs of Hematopoietic Stem Cell Transplantation Survivors
Multiple factors contribute to the development of late effects of disease or treatment for hematopoietic stem cell transplantation (HSCT) survivors. One of these factors is the accompanying treatment plan (chemotherapy and/or radiation). Recently, nonmyeloablative or reduced-intensity preparative regimens have been used; these regimens produce fewer acute toxic effects and can be used in elderly patients and those with comorbidities. The incidence and severity of late effects depend on cumulative exposure to cytotoxic therapies, infectious complications, and, with allogeneic transplants, the effects of graft-versus-host disease (GVHD). Age and the presence of comorbid conditions also impact late effects. For these reasons, HSCT survivors deserve early and ongoing education about known late effects, guidance about prevention and healthy lifestyle behaviors, lifelong monitoring, and immediate evaluation and management of potential late effects.
At MD Anderson, we have initiated a survivorship program designed to address the unique needs of HSCT survivors, including disease surveillance, monitoring for late effects, risk reduction, early detection, and attention to psychosocial functioning. HSCT survivors are also at risk to develop other medical problems that may be independent of their cancer treatment, such as new cancers or cardiovascular or degenerative diseases. An HSCT survivorship program nurse practitioner visit accompanies follow-up with the primary HSCT physician. Visits are scheduled for the same or next day as the survivor’s HSCT physician follow-up appointment and occur at critical points along the survivorship continuum, beginning at around 100 days after infusion of stem cells and then at 6, 12, 18, and 24 months, and annually thereafter. The deliberately separate clinic visit shifts the focus of the survivorship clinic visit from disease management to “survivorship.” At the identified time point above, HSCT survivors are screened and if indicated receive treatment and guidance from experts in nutrition, behavioral science, neurocognitive science, vocational counseling, psychiatry, sexuality, fertility, cancer prevention services, bone health, infectious disease, endocrinology, pulmonology, cardiology, nephrology, and other organ systems. HSCT survivors have a lifelong need for monitoring of potential late effects as an integral part of the education and planning that they receive, and individualized survivorship plans will soon to be available electronically in each survivor’s medical record. These plans can guide all health care providers regarding lifelong survivorship care. A review of the most common late effects that may impact the health of survivors and our plan for monitoring follows.
Physiologic Late Effects
Secondary Malignancy
HSCT survivors are at high risk of developing secondary malignancies, most commonly myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), solid tumors, and posttransplant lymphoproliferative disorders.
The risk for secondary malignancies is significantly higher than in the general population for both autologous and allogeneic HSCT survivors (Tichelli et al. 2009). This is related to the cumulative exposure to cytotoxic agents. Exposure to radiation, either through total body irradiation (TBI) or limited-field radiation therapy, is the most significant risk factor for secondary malignancies occurring more than 5 years after completion of treatment.
Risk factors for MDS/AML include pretransplant treatment with alkylating agents (e.g., cyclophosphamide, melphalan, carmustine, busulfan, dacarbazine, thiotepa, and temozolomide), treatment with topoisomerase II inhibitors (e.g., doxorubicin, epirubicin, etoposide, mitoxantrone, and amsacrine), limited-field radiation therapy, TBI, and autologous HSCT. Development of MDS/AML most frequently occurs 2–5 years after autologous HSCT (Majhail 2008). Therapy-related MDS/AML is rare after allogeneic HSCT.
Common solid tumors occurring after HSCT include thyroid cancers; squamous cell cancers of the head, neck, and vulva; breast cancer; melanoma; and non-melanoma skin cancer. The most common of these are basal-cell skin cancers and central nervous system tumors (Schwartz et al. 2009). The incidence of solid tumors is calculated to be 1% in allogeneic HSCT survivors living 10 years after HSCT and 2.2% in allogeneic HSCT survivors living 15 years after HSCT (Majhail et al. 2011). Risk factors for solid tumors include TBI, chronic GVHD, and prolonged immunosuppression. Cutaneous melanoma generally has a latent period of 1 year or less, and radiation therapy and T-cell depletion are cited as contributing factors (Rizzo et al. 2009).
Posttransplant lymphoproliferative disorders are related to Epstein-Barr virus infection and usually occur within the first 6 months after HSCT. The incidence is 1–6%, and the disorders tend to occur in the most severely immunocompromised patients; risk factors include T-cell–depleted grafts, use of antithymocyte globulin, and unrelated or human leukocyte antigen (HLA)-mismatched donor grafts (Majhail 2008; Rizzo et al. 2009).
The risk for secondary malignancies continues as survivors age. Each survivor should be well educated about self-examination and the importance of yearly physical examinations and frequent dental examinations, which screen for head and neck cancers. Careful examination of oral mucosal surfaces with each dental evaluation and at yearly follow-up visits with a health care professional should be performed. Annual physical examinations should include a complete blood count, as well as palpation of the neck, thyroid, and lymph nodes and examination and palpation of the skin, breast, and testicles. Adherence to the American Cancer Society and American Society for Blood and Marrow Transplantation cancer screening guidelines is recommended, and some may recommend starting screening at an early age, such as beginning annual mammograms at an earlier age in patients who have received radiation therapy to the chest (Majhail et al. 2011).
Ocular Late Effects
The most common ocular effects are keratoconjunctivitis sicca (dry eye syndrome), cataracts, and retinopathy.
Dry eye syndrome is defined as the deficiency of tears or the evaporation of tears. About 50% of HSCT survivors develop dry eye syndrome by 6 months after HSCT. Forty percent of HSCT survivors with chronic GVHD experience dry eye syndrome, compared with 10% of HSCT survivors without GVHD. Risk factors for dry eye syndrome include TBI, use of methotrexate for GVHD prophylaxis, peripheral blood stem cell grafts, and chronic GVHD (Socie et al. 2003). Dry eye syndrome can occur in different stages. Symptoms are dry, gritty, sandy feeling or irritation in the eyes. A Schirmer test result of <5 mm is helpful in objectively identifying dry eye syndrome. Dry eye syndrome may contribute to damage to the ocular surface and increased risk for infection, which may lead to loss of vision. Survivors who are diagnosed with dry eye syndrome may further progress to a sicca syndrome that is characterized by dry mouth, dry skin, dry eyes, and vaginitis. Treatments for dry eye syndrome include preservative-free ocular lubricants, punctual occlusion, topical steroids, other topical immunosuppressive agents, and autologous serum tears (Leite et al. 2006). An ophthalmologist familiar with the ocular changes that HSCT survivors experience can also make recommendations for other products to improve symptom management.
A cataract is a clouding of the lens or the eye that becomes very dense and significantly impairs vision as it develops over time. Symptoms include blurry vision, double vision, sensitivity to light, and poor night vision. The most significant risk factors for cataract development in HSCT survivors are glucocorticoid use (longer than 3 months) and TBI. Patients who received 10-Gy single fractions of TBI are likely to develop cataracts by 4 years after treatment. About 80% of patients who received fractionated TBI were found to have cataracts by 6–10 years after HSCT (Socie et al. 2003). Early-stage cataracts are usually closely monitored and the patient is encouraged to use improved lighting, corrective lenses, glare-protection lenses, sunglasses, or magnifying lenses as appropriate for symptom management. As the cataract matures or ripens, surgical intervention to remove the affected lens and replace it with an artificial lens is generally required.
Retinal hemorrhage is described as the most frequent retinal complication of HSCT, with an incidence rate of 3.5–20% in survivors. Risk factors include GVHD-related vasculopathy, cytomegalovirus retinitis, and recurrence of leukemic diseases (Socie et al. 2003).
Ophthalmologic screening recommendations for ocular late effects include yearly review of ocular symptoms, dilated fundoscopic examination by an ophthalmologist, and visual acuity testing and tonimetry (ocular pressure testing). Survivors should be educated about the need for immediate evaluation by an ophthalmologist if sudden visual disturbance occurs. Frequently occurring or chronic visual disturbances should also prompt an ophthalmologic examination.
Oral Late Effects
The most common oral effects are dry mouth and increased prevalence of dental caries.
Dry mouth is decreased saliva production after HSCT. Dry mouth can be caused by damage to salivary glands as a result of chemotherapy, radiation therapy, and GVHD. This alters the oral environment, resulting in changes in oral tissues and an imbalance of chemicals and microbes, which often results in dental caries. Decreased saliva production may also be a side effect of common drugs used by the HSCT patients, such as antiemetics and antidepressants. As mucosal cells regenerate after HSCT and patients stop taking the offending medications, many report improvement in saliva production. Risk factors for ongoing dry mouth are TBI or radiation to the head and neck area and GVHD. In patients who have received radiation (either TBI or to the head and neck area), dry mouth may be a lifelong problem (Dobr et al. 2007). Review of oral symptomatology and assessment of the oral cavity should be performed at each follow-up visit. The finding of dry mouth can be important in the diagnosis of chronic GVHD and sicca syndrome, which may include dry skin, dry eyes, and vaginitis. Dry mouth may affect the patient’s ability to taste, chew, and swallow. Counseling regarding use of beverages before and liberally during meals, addition of gravies and sauces on foods, and use of artificial saliva products may alleviate some of these issues. If dry mouth is associated with GVHD, systemic or topical treatment may also relieve dry mouth. Over-the-counter, alcohol-free oral moisturizers are readily available for dental and oral hygiene to soothe dry mouth and improve associated halitosis.
The incidence of dental caries is increased in patients who have extended symptoms of dry mouth and in patients with oral chronic GVHD. Prevention measures include good oral hygiene practices and daily use of fluoride rinses or gels, which improve the resistance of hard dental surfaces to bacterial acids and bacterial growth. Chlorhexidine mouthwashes can contribute to reduction in accumulation of plaque (Dobr et al. 2007).
Dental visits are recommended every 6–12 months for monitoring and restorative intervention of dental caries as well as thorough examination of the oral mucosa to check for suspicious lesions that may be early cancers.
Endocrine Late Effects
Thyroid and reproductive late effects are the most common endocrine-related late effects. Corticosteroid treatment for GVHD is commonly complicated by diabetes mellitus.
Thyroid dysfunction is one of the most common late sequelae of HSCT. Because development of thyroid disorder can predispose HSCT survivors to cardiac and metabolic disease, ongoing monitoring for thyroid dysfunction is important. The thyroid disorders most frequently seen are subclinical hypothyroidism, overt hypothyroidism, and, less commonly, autoimmune thyroid disease (Roziakova and Mladosievicova 2010).
About 30% of HSCT survivors develop subclinical compensated hypothyroidism and 15% develop overt primary hypothyroidism. The median time to diagnosis is reported to be about 50 months (Socie et al. 2003). Risk factors include treatment with 10-Gy single-dose TBI, which is associated with the highest incidence of hypothyroidism, as well as fractionated TBI and treatment with busulfan. Other chemotherapies, as well as prolonged chronic GVHD, are also risk factors.
Subclinical hypothyroidism is a compensated, benign, and most often a temporary finding in laboratory tests showing slightly increased thyroid-stimulating hormone (TSH) levels and normal T4 levels. Treatment of this condition is controversial because treatment can put patients at risk for problems such as osteoporosis or iatrogenic hyperthyroidism. Conversely, early treatment may decrease the risk of thyroid adenoma or carcinoma and prevent growth problems or delayed development in pediatric patients. Certainly if subclinical hypothyroidism persists or increases over many months, treatment should be considered.
Laboratory diagnosis of overt primary hypothyroidism shows high serum TSH levels and low concentrations of free T4. Many of the symptoms that are common with hypothyroidism are also commonly related to other effects of treatment, and thus laboratory testing should be used for confirmation. These symptoms include fatigue, weakness, weight gain, constipation, depression, memory loss, dry rough skin, coarse dry hair, irritability, decreased libido, muscle cramps or aches, abnormal menstrual cycles, and cold intolerance.
An uncommon but documented thyroid disorder, autoimmune thyroid disease, may occur as a late effect of HSCT. Autoimmune thyroid disease includes autoimmune thyroiditis and autoimmune-mediated hyperthyroidism. Autoimmune thyroiditis symptoms include the previously listed symptoms of hypothyroidism. Hyperthyroid symptoms include enlarged thyroid, nervousness, irritability, tremors, weight loss, sweating, palpitations, diarrhea, excessive tearing, double vision, pretibial myxedema, and exophthalmos.
Annual screening of laboratory TSH and free T4 levels is recommended. If subclinical hypothyroidism is noted, laboratory screening every 6 months should be considered until the decision is made to treat. A physical examination, including height and weight check and hair, skin, and thyroid examination, should be performed. More frequent screening and examination during periods of rapid growth should be considered in children. Treatment for hypothyroidism includes oral administration of L-thyroxine. Treatment for hyperthyroidism may require medication or radiation therapy to regulate or ablate the thyroid function.
Gonad and ovarian failure are known sequelae of treatment. Many HSCT survivors have undergone various modes and courses of treatment prior to HSCT, which contributes to poor fertility. The number of conceptions after autologous or allogeneic HSCT is low. However, if conception does occur, the likelihood of a live birth is favorable (Loren et al. 2011). Very few women who are conditioned with busulfan or TBI experience gonad recovery, and a low rate of gonad recovery has been observed in men conditioned with TBI. Among men who are conditioned with busulfan, about 17% show gonad recovery (Socie and Tichelli 2004).
Pretransplant strategies to preserve fertility (i.e., tissue/ova preservation or sperm banking) should be discussed with the patient and initiated if prior therapy, patient preference, and time before treatment allows. Posttreatment medical evaluation of hormone production and sperm analysis should be done to confirm infertility at 1 year or more after HSCT. Counseling should be provided regarding the use of birth control measures until confirmation of infertility can be made 1 or more years after HSCT. Fertility testing may be recommended at various time points after HSCT. Consultation with fertility specialists can be helpful for young adults, even after HSCT, to review all possible options.
The common use of corticosteroids to treat GVHD in allogeneic HSCT recipients frequently leads to hyperglycemia or diabetes mellitus. These conditions require close monitoring and treatment; they usually improve but may not resolve when corticosteroids are discontinued.
Skeletal Late Effects
Low bone mass as evidenced by osteopenia or osteoporosis, detected by bone mineral density testing, occurs in up to 50% of HSCT survivors by 12–18 months after HSCT. Osteopenia, with a t-score of −1 to −2.5, may occur in up to 30% of HSCT survivors, and osteoporosis, with a t-score of less than −2.5, occurs in about 10% of HSCT survivors. Nontraumatic fractures occur in 10% of HSCT survivors with low bone mass (Socie and Tichelli 2004). Risk factors for development of low bone mass include chemotherapy, radiation therapy, treatment with calcineurin inhibitors (tacrolimus, cyclosporine A), treatment with glucocorticoids (increasing with total dose and duration of therapy), hypogonadism, and nutritional and lifestyle factors (Socie et al. 2003). Preventive measures include sex hormone replacement therapy for those with hypogonadism or premature ovarian failure, oral supplementation of vitamin D, calcium supplementation using calcium-rich food sources and additional supplementation as needed, physical exercise that includes weight-bearing and resistance exercise, tobacco cessation, and moderation of alcohol intake. The use of bisphosphonate therapy for adults whose treatment with glucocorticoids is anticipated to last more than 3 months and for those with osteopenia is currently being studied.
Recommendations to avoid sun exposure and use sunscreens make vitamin D deficiency a common problem. This is a major issue for most allogeneic HSCT survivors. Vitamin D levels are checked using the 25-OH vitamin D test. If the vitamin D levels are lower than 20 ng/mL, vitamin D replacement using prescription oral ergocalciferol for 8–12 weeks followed by over-the-counter oral vitamin D supplementation of 800–1,000 international units per day is recommended. The baseline study of bone mass is obtained by bone mineral density study (dual photon densitometry) conducted at 6 months after HSCT. This study is repeated at 24 months after HSCT, unless the patient has a clinical reason to follow up earlier. HSCT survivors with osteopenia or osteoporosis, those receiving extended immunosuppressive therapy, or those with ovarian or gonad failure without hormone supplementation are instructed to undergo regular bone density studies.
Treatment is considered for HSCT survivors with a diagnosis of osteoporosis or high-risk osteopenia after evaluation of risk in regard to comorbid conditions, current clinical condition, and current drug therapy. Resumption of therapy for HSCT survivors for whom it was discontinued during the transplantation period should be directed by the primary HSCT physician.
Avascular necrosis (AVN) is the death of part of a bone because of an impaired blood supply. The incidence of AVN varies from 4% to >10% of HSCT survivors (Socie and Tichelli 2004). Timing is not well defined. Risk factors for AVN include TBI and cumulative exposure to corticosteroids. Incidence of AVN has been shown to be higher among HSCT survivors who received 10-Gy single-dose TBI than among those treated with fractionated TBI. AVN can lead to cracks in the affected bone and bone collapse. The femoral head is affected in 80% of cases of AVN (Socie et al. 2003). The wrist, shoulder, and knee are also frequently affected. Bones in the foot, ankle, spine, or jaw may be affected, but less frequently. The most common symptom is pain. If the hip is affected, pain is associated with standing and walking, and it is noted in the hip or groin and may radiate from thigh to knee. If the wrist is affected, pain in the wrist and weakness of the fingers may be noted. Shoulder pain and stiffness may be symptoms of shoulder involvement. Knee pain requires investigation.
Report of related symptoms should lead to further investigation. The diagnosis of AVN is best made by magnetic resonance imaging of the affected bone. A positive diagnosis warrants referral to an orthopedic specialist. Treatment in the early stage can include pain control and orthopedic measures to relieve pressure on the affected area. As AVN progresses, surgical replacement of the joint and affected bone is likely required.
Pulmonary Late Effects
Pulmonary effects in autologous HSCT survivors are generally related to chemotherapy and radiation lung toxicity and generally occur within 3 months after the end of treatment (Tichelli et al. 2008). Allogeneic HSCT survivors are more frequently affected by serious pulmonary late effects than are autologous HSCT survivors. Both infectious and noninfectious late effects can occur and have serious consequences. Air flow obstructive disorders are associated with the highest mortality rates. The most frequently occurring late-onset pulmonary complications are bronchiolitis obliterans syndrome (BOS), cryptogenic organizing pneumonia, sinopulmonary infections, and idiopathic pneumonia syndrome, also known as interstitial pneumonitis. These late-onset pulmonary effects are usually noted in the 6- to 12-month period after HSCT, but new onset has been reported 2–3 years after HSCT, and as many as 40% of allogeneic HSCT recipients may be affected.
BOS is the most common and lethal of air flow obstructive disorders. It is known to occur most frequently in HSCT recipients with GVHD, but cases of airflow obstruction disorders, specifically BOS, have also been reported in a small percentage of HSCT recipients who did not have other signs of GVHD (Pandya and Soubani 2010). Other risk factors include age >20 years at time of treatment, presence of pretransplant air flow obstruction (FEV1/FVC < .7), and viral respiratory infections within the first 100 days after HSCT (Dudek and Mahaseth 2006). The toxicity of chest irradiation, TBI, and chemotherapy, especially thiotepa and busulfan, is implicated in the development of pulmonary late effects. Because survivors who are diagnosed with serious pulmonary late effects have higher mortality rates than survivors who are not diagnosed with pulmonary late effects, close monitoring is recommended. Eighty percent of cases of BOS are noted between 6 and 12 months after HSCT. BOS that develops in the first 200 days after HSCT is associated with a worse prognosis than BOS that develops later (Patriarca et al. 2009). Progressive decline in FEV1 (≥20%) or FEV1/FVC < .7 with or without symptoms heightens the suspicion of BOS, and prompt pulmonary evaluation with a high-resolution computed tomographic scan and a transplantation center pulmonary service consultation should be considered. Often pulmonary function tests may not meet suggested criteria for BOS even though the patient presented with recent upper respiratory infection, wheezing, dry cough, and dyspnea. A chest x-ray may appear normal. In the absence of an infectious process, further evaluation for early airflow obstructive disorder should be initiated. An annual physical examination should include a review of pulmonary symptoms and a clinical chest examination (see Fig. 10.1 for screening recommendations).
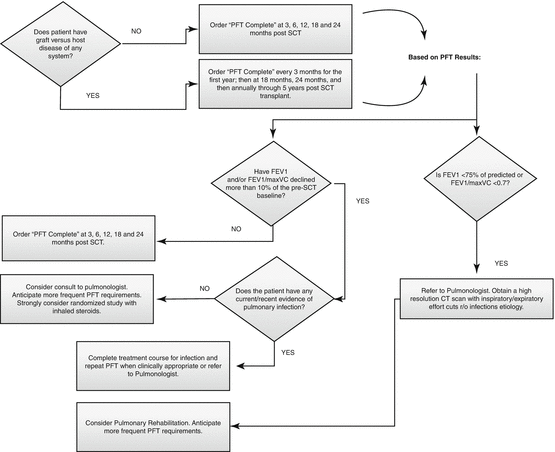

Fig. 10.1
Pulmonary screening recommendations for survivors of hematopoietic stem cell transplantation (PFT indicates pulmonary function test, SCT stem cell transplantation)
Cardiovascular Late Effects
A broad range of cardiovascular late effects may occur, including coronary and peripheral arterial disease, cardiomyopathy, arrhythmia, autonomic neuropathy, and cerebrovascular events. Metabolic syndrome can also occur.
Survivors of HSCT are at increased risk for early cardiovascular events compared with the general United States population. Late cardiac effects occur more frequently in patients who have had allogeneic HSCT than in patients who have had autologous HSCT. Chemotherapy, radiation therapy, and GVHD can cause direct damage to the vascular or arterial endothelium, contributing to the development of atherosclerotic lesions. Anthracyclines and mediastinal radiation can cause direct cardiac damage. Other risk factors include arterial hypertension, dyslipidemia, endocrine disorders, prolonged steroid use, and iron overload (Tichelli et al. 2007). Metabolic syndrome, which increases the absolute risk for cardiovascular disease in the general population, should be considered when developing a risk profile for the HSCT survivor (Grundy et al. 2005).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree