Grade
Brock, 1991 (Brock et al. 1991)
Muenster classification, 2007 (Schmidt et al. 2007)
National Cancer Institute CTCAE v. 4.0, 2010 (National Cancer Institute: Common Terminology Criteria for Adverse Events, Version 4.03)
0
<40 dB at all frequencies
<10 dB at all frequencies
<20 dB at 1000; 2000; 4000 Hz
≤20 dB HL at all frequencies
1
>40 dB loss at 8000 Hz
>10 dB to <20 dB loss at all frequencies
1a >40 dB at any freq 6000–12,000 Hz
Shift or loss of 15–25 dB, two freq, one ear
>20 dB HL (i.e., 25 dB HL or greater); SNHL above 4000 Hz (i.e., 6 or 8 kHz)
1b >20 and <40 dB at 4000 Hz
2
>40 dB loss at 4000 Hz and above
2a >20 to <40 dB loss at >4000 Hz
2a ≥40 dB at 4000 Hz and above
Shift or loss of 25–90 dB two freq, one ear
>20 dB HL SNHL at 4000 Hz and above
2b >40 to <60 dB loss at >4000 Hz
2b >20 and <40 dB at any freq below 4000 Hz
2c >60 dB loss at >4000 Hz
3
>40 dB loss at 2000 Hz and above
3a– >20 to <40 dB loss at <4000 Hz
≥40 dB at 2000 or 3000 Hz and above
>20 dB bilateral or >30 dB unilateral loss in speech frequencies or use of hearing device
>20 dB HL SNHL at 2000 Hz or 3000 Hz and above
3b– >40 to <60 dB loss at <4000 Hz
3c- >60 dB loss at <4000 Hz
4
>40 dB loss at 1000 Hz and above
>80 dB loss at <4000 Hz
≥40 dB at 1000 Hz and above
Audiologic indication for cochlear implant
>40 dB HL (i.e., 45 dB HL or more); SNHL at 2000 Hz
There are three grading scales that are not appropriate for reporting hearing loss. Radiation Therapy Oncology Group criteria considers subjective hypoacusis as an adverse effect, and it does not require the distinction between conductive and SNHL (Cox et al. 1995). The European Organization for Treatment of Cancer does not include the category “ear” in late adverse effects (Cox et al. 1995). Late Effects Normal Tissue Task Force Subjective, Objective, Management, and Analytical scale includes objective and analytical evaluation tools, but the use of an audiogram is up to the discretion of the treating physician (Mujica-Mota et al. 2013; Pavy et al. 1995).
33.5 Consequences
Hearing loss has an impact on speech understanding, academic performance, socialization, and quality of life. Mild bilateral hearing loss of 15–25 dB can have an impact on academic performance, and roughly 40% of children with this degree of hearing loss may have to repeat a grade (Bess et al. 1998). Overall, cross-sectional analysis of survivors childhood brain tumors have a greater need for special education, lower college attendance rates, fewer close friends, and higher unemployment rates than age-matched controls (Gurney et al. 2009). The exact role that hearing loss plays in these children is unclear from this type of survey.
Children with clinically significant hearing loss should be evaluated by an audiologist and assessed for assistive listening devices or hearing aids (Lafay-Cousin et al. 2013). Appropriate amplification is important for speech development and language acquisition. In the regular school setting, use of preferential seating or an FM system may help children with hearing loss. Children with learning deficits might require special schooling with more direct or one-on-one teaching.
33.6 Therapeutic Interventions
33.6.1 Amifostine
Amifostine is an organic thiophosphate compound with cytoprotective properties used to prevent hematologic, renal, neural, and mucosal complications of chemotherapy without attenuating antitumor effects (Kemp et al. 1996; Glover et al. 1986, 1987). It is administered before and during cisplatin infusion as a twice daily dose of 600 mg/m2. It has a very short half-life of just 8 min, and infusions must be timed immediately before cisplatin administration. Amifostine can cause hypocalcemia, and close calcium monitoring is required. Amifostine has been studied in children with average risk medulloblastoma and found to be effective at reducing the risk of severe (grade 3 or 4) ototoxicity (Fouladi et al. 2008). The active metabolite of amifostine, WR-1065, acts as a scavenger of ROS and binds to platinum agents to prevent and reverse DNA platination (Treskes et al. 1992).
Long-term hearing loss following radiation and cisplatin has been reported (Knight et al. 2005; Merchant et al. 2004; Bertolini et al. 2004). Amifostine seems to protect against cisplatin ototoxicity over time, perhaps for as long as 3 years (Fouladi et al. 2008).
33.6.2 Steroids
Drugs can be administered as intratympanic therapy to avoid any potential systemic effect. Intratympanic administration has been used successfully for many years to deliver relatively high doses of steroids to the inner ear, by way of absorption or diffusion through the round window membrane. Steroids have been studied experimentally to identify a role for preventing radiation or chemotherapy ototoxicity. Early results show some promise, but this mode of therapy has not been adopted for widespread use.
33.6.3 Hearing Aids
The role of hearing aids has been described under Consequences.
33.6.4 Cochlear Implants
Cochlear implants in children have a long history of success for many different causes of hearing loss. There are published reports of children receiving cochlear implants after treatment for rhabdomyosarcoma (Torkos et al. 2002), medulloblastoma (Roland et al. 2010), and nasopharyngeal carcinoma (Chua and Tan 2007). Success rates for hearing rehabilitation seem to be equal between irradiated and nonirradiated ears (Soh et al. 2012). Even ears affected with osteoradionecrosis have been successfully implanted with good speech recognition (Adunka and Buchman 2007).
33.7 Prevention
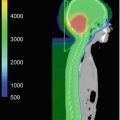
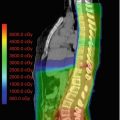
Limiting the radiation dosage to the cochlea is the best form of prevention of hearing loss. Improved target localization using fiducials or daily imaging might reduce set uncertainty and decrease the change that the cochlea is included in the target volume (Hua et al. 2008). Highly conformal planning and delivery techniques, such as IMRT or proton beam therapy, can also help to minimize cochlear dose.
Close monitoring of dose and hearing levels is important when cisplatin is being prescribed. Dose modification or drug elimination is required when significant hearing impairment is reached. While cisplatin has a proven track record of efficacy for many tumors, the search for targeted agents is prompted by the hope of avoiding side effects, such as ototoxicity or nephrotoxicity. Until new, effective chemotherapeutics agents are developed, children with brain tumors will still suffer hearing loss when cisplatin is delivered along with radiotherapy.
33.8 RT Dose Tolerance Guidelines
Information on the tolerance dose to the cochlea in children are largely lacking. Most information comes from adult studies that are extrapolated to children. The tolerance dose to the cochlea with a probability of 5% complication within 5 years has been reported to be 60 Gy (Emami et al. 1991). The tolerance dose to the cochlea with a probability of 50% complication rate within 5 years is 70 Gy (Emami et al. 1991). These figures might need to be reevaluated in the light of more recent studies, especially taking into account the effect of concomitant chemotherapy.
Pan et al. observed significant hearing loss (≥10 dB change) at 2 kHz and higher frequencies in adult patients when the dose was 45 Gy or greater (Pan et al. 2005). Chen et al. reported that dosages higher than 48 Gy are associated with hearing loss at 4 kHz in the majority of adult patients when radiotherapy is given with chemotherapy (Chen et al. 2006).
The threshold dose in children for auditory toxicity after radiation is reported to be within the range of 35–45 Gy (Merchant et al. 2004; Hua et al. 2008). Dosages less than 30 Gy have little effect on hearing (Merchant et al. 2004; Linskey and Johnstone 2003; Hua et al. 2008). Consistent with this fact is the finding from Thibadoux et al. who studied 61 children with acute lymphoblastic leukemia who received 24 Gy of craniospinal radiation but no chemotherapy. None of these children developed hearing loss at 3 years (Thibadoux et al. 1980). The dose threshold might be lower in children with supratentorial tumors and ventriculoperitoneal (VP) shunts, perhaps as low as 30 Gy (Merchant et al. 2004).
The TD50/5 for acute reactions of the middle ear has been estimated to be 40 Gy and for chronic otitis media to be 65–70 Gy (Emami et al. 1991). Wang et al. reported that the rate of middle ear effusions could be diminished if the dose to the middle was kept under 34 Gy and the dose to the isthmus of the Eustachian kept under 53 Gy (Wang et al. 2009). In an earlier study, Wang et al. demonstrated that the rate of otitis media with effusion was related to radiation dose given to the isthmus of the Eustachian tube (Wang et al. 2007). When dose to the isthmus was kept below 52 Gy and the dose to the middle ear was kept below 46 Gy, the rate of otitis media with effusion was decreased in their study population of nasopharyngeal patients (Wang et al. 2007).
References
Adunka OF, Buchman CA (2007) Cochlear implantation in the irradiated temporal bone. J Laryngol Otol 121(1):83–86. doi:10.1017/S0022215106002180 CrossrefPubMed
Asenov DR, Kaga K, Tsuzuku T (2007) Changes in the audiograms of a nasopharyngeal cancer patient during the course of treatment: a temporal bone histopathological study. Acta Otolaryngol 127(10):1105–1110. doi:10.1080/00016480601127026 CrossrefPubMed
Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, Hartmann O (2004) Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol 26(10):649–655Crossref
Bohne BA, Marks JE, Glasgow GP (1985) Delayed effects of ionizing radiation on the ear. Laryngoscope 95(7 Pt 1):818–828PubMed
Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput K, Sullivan M, Neuwelt EA (2012) Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 30(19):2408–2417. doi:10.1200/JCO.2011.39.1110 CrossrefPubMedPubMedCentral
Chang KW, Chinosornvatana N (2010) Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol 28(10):1788–1795. doi:10.1200/JCO.2009.24.4228 CrossrefPubMed
Chao C, Saito S, Anderson CW, Appella E, Xu Y (2000) Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc Natl Acad Sci U S A 97(22):11936–11941. doi:10.1073/pnas.220252297 CrossrefPubMedPubMedCentral
Chen WC, Jackson A, Budnick AS, Pfister DG, Kraus DH, Hunt MA, Stambuk H, Levegrun S, Wolden SL (2006) Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer 106(4):820–829. doi:10.1002/cncr.21683 CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree