Location
Overall pooled mean CLABSI
Pooled mean CLABSI—permanent central line days
Pooled mean CLABSI—temporary central line days
Adult general medical/surgical inpatient
0.8
NA
NA
Adult medical/surgical ICU
0.8–1.1a
NA
NA
Adult general hematology-oncology ward
NA
1.4
2.0
Adult HSCT ward
NA
2.6
3.0
Pediatric general medical/surgical inpatient
0.9
NA
NA
Pediatric medical/surgical ICU
1.2
NA
NA
Pediatric general hematology-oncology ward
NA
2.1
2.1
Pediatric HSCT ward
NA
2.4
2.2
Microorganisms colonize most central lines, often in as a little as a day, by (a) migration from the skin insertion site along the external surface of the catheter, (b) introduction into the hub lumen during manipulation of the catheter or by exposure to contaminated infusates, or (c) hematogenous spread from a focal infection [3]. Thrombin covering the intravascular portion of the catheter and the biofilm produced by many microbial pathogens promotes the adhesion of pathogens. The risk of subsequent bloodstream infection is dependent on both the number of organisms and their intrinsic virulence. Host, underlying disease, and treatment characteristics also contribute to the risk of CLABSI [4, 5].
Many institutions in the United States monitor rates of inpatient central line infections and assess the effectiveness of prevention efforts through the Centers for Disease Control and Prevention’s NHSN, and these data are publically reported in many states. These surveillance strategies have also been applied to infections in ambulatory pediatric hematology and oncology patients [4, 6]. Specific criteria for bloodstream infections developed by the CDC Prevention Healthcare Infection Control Practices Advisory Committee (Table 11.2) distinguish between a CLABSI, an infection occurring in a patient who has had a central line in place for >2 days, and a catheter-related bloodstream infection (CRBSI), a CLABSI for which specific laboratory testing has identified the central line as the source of the infection [7]. Practically, it is sometimes not possible to implicate or exclude the catheter because the appropriate laboratory test was not feasible (e.g., if the central line is not removed and cultures of the catheter tip, therefore, not possible) or not obtained (e.g., simultaneous blood cultures from both the central line and a peripheral vein for comparison of time to positivity). The simpler definition of CLABSI has, therefore, been used for NHSN surveillance although it is recognized that it is less specific than desirable.
Table 11.2
Criteria for catheter and mucosal barrier injury laboratory-confirmed bloodstream infections
Laboratory-confirmed bloodstream infection (LCBI) | (1) A recognized pathogen identified from ≥1 blood cultures (2) Fever, chills, or hypotension in association with the same common commensal bacteria being obtained from ≥2 blood cultures drawn on separate occasions (3) Fever, hypothermia, apnea, or bradycardia in a patient ≤1 year of age in association with the same common commensal bacteria being obtained from ≥2 blood cultures drawn on separate occasions In each case, the organism identified from blood should not be related to an infection at another site (i.e., the infection represents a primary bacteremia), and criterion elements must take place 3 days before to 3 days after the collection date of the first positive blood specimen |
Central line-associated bloodstream infection (CLABSI) | A LCBI that develops in a patient with a central line in place for >2 days before the onset of the infection |
Catheter-related bloodstream infection (CRBSI) | A LCBI with additional laboratory evidence that identifies the central line as the source of the bloodstream infection (e.g., differential time to positivity of blood cultures) |
Mucosal barrier injury LCBI | A LCBI: (1) That meets LCBI criteria 1 and ≥1 blood specimen is positive for a select group of recognized intestinal organisms, in association with: a. A history of allogeneic HSCT within 1 year and Grade III or IV gastrointestinal GVHD) or ≥1 liter diarrhea (≥20 mL/kg in patients <18 years of age) in a 24-h period on or ≤7 days before the collection date of the first positive blood specimen b. A history of ≥2 days of an ANC or WBC <500 cells/mm3 on or within 3 days of the collection date of the first positive blood specimen (2) That meets LCBI criteria 2 and ≥1 blood specimen is positive for viridans group streptococci only, in association with: a. A history of allogeneic HSCT within 1 year and Grade III or IV gastrointestinal GVHD or ≥1 liter diarrhea (≥20 mL/kg in patients <18 years of age) in a 24-h period on or ≤7 days before the collection date of the first positive blood specimen b. A history of ≥2 days of an ANC or WBC <500 cells/mm3 on or within 3 days of the collection date of the first positive blood specimen (3) A patient ≤1 year of age who meets LCBI criteria 3 and ≥1 blood specimen is positive for viridans group streptococci only, in association with: a. A history of allogeneic HSCT within 1 year and Grade III or IV gastrointestinal GVHD or ≥1 liter diarrhea (≥20 mL/kg in patients <18 yrs. of age) in a 24-h period on or ≤7 days before the collection date of the first positive blood specimen b. A history of ≥2 days of an ANC or WBC <500 cells/mm3 on or within 3 days of the collection date of the first positive blood specimen |
Figure 11.1 the successes of early efforts to track, report, and prevent CLABSI over the last decade led to the emergence of a “zero tolerance” attitude toward CLABSI, with many organizations setting a goal of eliminating all infections through a series of interventions that include strict adherence to hand hygiene, asepsis during catheter insertion, adherence to a maintenance bundle, and the use of an appropriate dressing [8]. More recently, it has been recognized that many bloodstream infections in persons with cancer or severe neutropenia from other causes, or who have undergone HSCT, are not CRBSI, but result from the translocation of gastrointestinal microorganisms to the bloodstream, particularly in patients with severe neutropenia or who have gastrointestinal graft versus host disease [9]. Use of CLABSI as a surveillance definition in these populations, therefore, overestimates the proportion of bloodstream infections that are attributable to central lines and has implications for whether or not these infections may be prevented by traditional approaches or, indeed, if these infections are preventable at all [10]. In 2013, the NHSN introduced a new surveillance definition of “mucosal injury-associated laboratory-confirmed bloodstream infection” (MBI-LCBI, Table 11.2). Additional studies of the impact of distinguishing MBI-LCBI from CLABSI in high-risk pediatric and adult populations are ongoing.
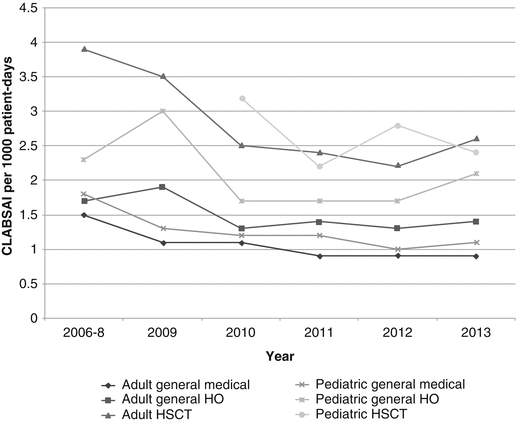

Fig. 11.1
Pooled mean rates of permanent central line-associated bloodstream infections (number of infections per 1000 patient-days) in hospitalized adults and children 2006–2013, by care unit. Data from the National Healthcare Safety Network, available at: http://www.cdc.gov/nhsn/datastat/index.html
Clostridium difficile Infection (CDI)
C. difficile is the single most common organism causing HAI in the United States, with an estimated incidence of 95.3 infections per 100,000 persons overall and 6.3 per 100,000 children under 18 years of age [11, 12]. C. difficile is a Gram-positive, anaerobic, spore-forming bacillus. Intestinal colonization occurs when infectious spores, which may persist for long periods in the environment, are ingested. Some strains elaborate two homologous exotoxins, toxin A and toxin B,which bind to and damage intestinal epithelial cells and incite strong inflammatory responses [13]. North American pulsed-field gel electrophoresis type 1 (NAP1) PCR ribotype 027 strains of toxogenic C. difficile, which express more toxin A and B than other strains and an additional binary toxin, emerged in the early 2000s. Now commonly found in North America, these strains are associated with more severe disease in adults, but it is not yet clear whether they are more pathogenic in either children or cancer patients [14].
In order for CDI to occur, patients must be colonized with toxogenic C. difficile and undergo some alteration in the gastrointestinal microbiome that promotes decreased microbial diversity [13]. The most commonly recognized risk factor for CDI is antibiotic use; others include antineoplastic chemotherapy, the use of proton pump inhibitors, gastrointestinal surgery, inflammatory bowel disease, and immunocompromising conditions [15]. Adults with cancer and recipients of HSCT are at significantly greater risk of CDI than the general hospital population, with rates ranging from 3.4% to 27% [16]. Disease manifestations range from mild diarrhea to fulminant pseudomembranous colitis. Complications such as toxic megacolon, bowel perforation, and sepsis are responsible for an estimated fatality rate of up to 15% in adults [12]. Malignancy is also a strong predictor of recurrent CDI. Most children with healthcare-associated CDI have underlying medical comorbidities, with malignancy being the most common. Up to 25% of cases of CDI in hospitalized children occur in those with cancer, a rate tenfold higher than that observed in children without cancer [17, 18]. Boyle reported 17% of pediatric HSCT recipients older than 1 year of age developed CDI within 100 days of transplant (20/10,000 patient days), significantly higher than that the rate observed in adult recipients [19]. Severe and complicated disease appear to be less common in children than adults, but more frequent in children with cancer than those without malignancy [15, 16, 20].
The NHSN defines healthcare-associated CDI as a positive test for toxin-producing C. difficile in an unformed stool specimen and/or gross anatomic or histopathological evidence of pseudomembranous colitis, with disease beginning >3 days after admission to a healthcare facility [21]. This and other clinical and surveillance definitions have significant limitations that may affect estimates of the incidence of CDI in pediatric oncology patients. Over half of young children, especially those <2 years of age, and almost a third of pediatric oncology patients may be asymptomatically colonized by C. difficile, often for long periods of time [22–24]. Some investigators have suggested that sensitive molecular diagnostic tests, such as nucleic acid amplification of toxin A and B genes, are more likely to overestimate the incidence of CDI than older assays because these are more likely to identify the clinically inconsequential carriage of C. difficile [22]. Viruses and other gastrointestinal co-pathogens are also detected in as many as 80% of children, including immunocompromised children with CDI, making it difficult to judge the contribution of each potential pathogen to diarrheal disease [25, 26]. Importantly, however, patients who are colonized with toxogenic C. difficile may still represent a source of environmental shedding and transmission of infectious spores. Judicious use of antimicrobials, infection prevention precautions, and environmental cleaning are the mainstays of CDI prevention in healthcare settings.
Ventilator-Associated Pneumonia (VAP)
The NHSN surveillance definition for pneumonia incorporates the results of diagnostic imaging, clinical signs and symptoms, and laboratory tests [27]. A specific algorithm is available for immunocompromised patients (Table 11.3). Ventilator-associated pneumonia (VAP) is defined as pneumonia that occurs >2 calendar days after a patient is placed on mechanical ventilation; the ventilator must have been in place on the day that the first criterion for the diagnosis of VAP was met or on the previous day.
Table 11.3
National Hospital Safety Network surveillance definition for pneumonia in immunocompromised patients
Evidence | Definition |
|---|---|
Diagnostic imaging | ≥2 serial chest imaging studies at a ≤ 7-day interval demonstrating at least one of: • New or progressive and persistent infiltrate(s) • Consolidation • Cavitation • Pneumatoceles in infants ≤1 year of age In patients without underlying pulmonary or cardiac disease, a single unequivocal chest imaging study is acceptable |
Clinical findings | At least one of: • Fever (>38.0 °C) • New onset of purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements • New onset or worsening cough, dyspnea, or tachypnea • Crepitations or bronchial breath sounds • Hemoptysis • Pleuritic chest pain |
Laboratory tests | At least one of: • Positive blood culture not related to another source of infection • Positive pleural fluid culture • Positive quantitative culture from minimally contaminated lower respiratory tract specimen • 5% BAL-obtained cells with intracellular bacteria on Gram stain • Positive quantitative culture of lung tissue • Histopathological evidence of abscess formation or intra-alveolar/bronchiolar accumulation of PMNs For viral and fastidious bacterial (e.g., Legionella) pneumonias: • Positive culture from respiratory secretions • Positive nonculture diagnostic test from respiratory secretions • Fourfold rise in paired acute and convalescent serum antibody titers • Detection of Legionella antigen in urine For fungal infection: • Matching positive blood and sputum/ET aspirate cultures with Candida spp. • Evidence of fungi from minimally contaminated LRT specimen by direct microscopic examination and culture or nonculture diagnostic test |
The precise incidence and clinical outcomes of VAP have been difficult to establish because studies have used diverse diagnostic criteria and inconsistently applied these criteria and because diagnostic definitions for VAP, including those reported by the NHSN, have limited sensitivity and specificity [28]. The signs and symptoms of VAP, for example, may overlap with other infections, such as tracheobronchitis, and with noninfectious pulmonary disorders [29]. Reactivation of latent pulmonary or systemic infection, such as cytomegalovirus or tuberculosis, in oncology and transplant patients, including children, may be indistinguishable from VAP. Some features of the current NHSN surveillance definition make its application to children problematic. Respiratory specimens, for example, must be obtained by methods that limit contamination, such as bronchoalveolar lavage, that may have technical limitations and greater risks in young patients [30]. Recognizing the limitation of VAP surveillance definitions, NHSN surveillance began to assess a broader range of ventilator-associated events, including VAP, in adults in 2015 [31]. A pediatric-specific algorithm for VAP is not yet available, but one study that applied adult definitions retrospectively to PICU patients receiving mechanical ventilation suggests that this strategy may be useful [32].
Obstacles to diagnosis notwithstanding, VAP are the second most common HAI in pediatric and neonatal intensive care units. In the United States in 2012, there were 0.7 VAP per 1000 ventilator days in pediatric medical/surgical units [33]. Overall, between 3% and 10% of ventilated PICU patients develop VAP, a rate that is approximately threefold lower than that of adults (Table 11.4) [34]. Data are limited, but have suggested that VAP in children, as in adults, is associated with longer duration of mechanical ventilation and PICU stay, greater hospital costs, and increased mortality [35, 36]. The specific rates and characteristics of VAP in pediatric oncology patients have not been reported, but it is plausible that immunosuppression might predispose to infection and increase the rate and severity of VAP in this population.
Location | Pooledmean CAUTI |
|---|---|
Adult general medical/surgical inpatient | 1.3 |
Adult medical/surgical ICU | 1.3–2.7a |
Adult general hematology-oncology ward | 2.1 |
Adult HSCT ward | 2.2 |
Pediatric general medical surgical inpatient | 1.4 |
Pediatric medical/surgical ICU | 2.5 |
Pediatric general hematology-oncology ward | 3.0 |
Pediatric HSCT ward | 0.0 |
The pathogenesis of VAP has not been completely elucidated. VAP may, like conventional pneumonia, result from the inhalation of infectious aerosols or complicate hematogenous bacteremia or fungemia. The presence of the same microorganisms in the oropharynx and in endotracheal aspirates, however, suggests that a frequent and potentially preventable cause of VAP is the aspiration of microorganisms colonizing the endotracheal tube (ET), oropharynx, or stomach [37]. Micro-aspiration of upper airway secretions around the uncuffed ET commonly used in infants and children or through channels formed by folds in low-pressure high-volume cuffed ET may be exacerbated by the impairment of mucociliary clearance and pooling of secretions in the subglottic airway. Risk factors for VAP in children include a prolonged duration of mechanical ventilation, prior antimicrobial exposure, and the use of immunosuppressing drugs, particularly corticosteroids [38]. Additional contributing elements may include the replacement of the usual microbiological flora of the oropharynx and stomach by more virulent species (such as Staphylococcus aureus and Gram-negative bacilli), the contamination of suction equipment (particularly associated with the use of open systems and nonsterile solutions), and the presence of a nasogastric tube, enteral feeding, poor oral hygiene, gastric distension, and positioning (with the semirecumbent position associated with a lower risk of VAP than the supine position). The contribution of each of these elements to the pathogenesis of VAP in pediatric hematology-oncology patients has not been studied; most prevention strategies target multiple risk factors for infection.
Catheter-Associated Urinary Tract Infections (CAUTI)
Overall, urinary tract infections (UTI) are the fourth most common HCA infection in the United States [39]. Almost all are related to catheterization or other instrumentation. Short-term indwelling urinary catheterization may be necessary because of acute urinary retention or obstruction, or the need to monitor urinary output (especially perioperatively, during critical illness, or if receiving large volumes of fluid or diuretics). More prolonged use may be required to promote healing of sacral or perineal wounds or incisions, in incontinent patients or for comfort during end of life care.
The NHSN defines CAUTI as a UTI that occurs >2 calendar days after a urinary catheter is placed and <1 day after the catheter has been removed, if applicable [39]. Rates are generally higher in children than in adults (Table 11.4), but little has been reported on outcomes in children outside of the intensive care setting or in specific populations such as pediatric hematology-oncology and transplant recipients. CAUTI are associated with secondary bloodstream infections, increased hospital stay and costs and, in adults, increased mortality [40, 41].
Most CAUTI are ascending in origin. Uropathogens that colonize the periurethral area adhere to fibrinogen that accumulates on the catheter, multiplying and forming biofilm [41]. Thereafter, bacteria may colonize the bladder, often within days, releasing toxins and proteases that damage urinary epithelium and promote ascension to the kidney and hematogenous dissemination. Up to a third of infections may occur from contamination of the urinary collecting system from exogenous sources, such as the hands of healthcare providers [42]. Efforts to prevent CAUTI have focused on reducing the duration of catheter use and the contamination of drainage systems.
Respiratory Viral Infections
Most respiratory viruses are spread by indirect contact, droplet, or airborne transmission. Sources of infection include other patients, caregivers or visitors, and healthcare providers, who may be asymptomatic or symptomatic, and contaminated environmental sources [43]. Patient factors that increase the likelihood of healthcare-associated respiratory viral infections in pediatric hematology-oncology and transplant patients include their frequent close physical contact with caregivers, young age, lack of previous natural infection or immunization and subsequent acquired immunity, and the presence of primary or secondary immunodeficiency. In the ambulatory healthcare setting, patients may be cared for in extended periods in common areas; the risk of infection is increased when infectious persons are not immediately recognized and when environmental cleaning is inadequate because of time constraints.
Strategies to reduce healthcare-associated respiratory viral infections include transmission-based infection, prevention, precautions, and vaccination. Institutions must provide infection prevention staff, clinical microbiology support, and the supplies and equipment necessary to assess and correct remediable causes of healthcare-associated respiratory viral infections. Risk assessment should inform the development of processes for the surveillance for and management of endemic, epidemic (e.g., influenza), and emerging (e.g., Middle East respiratory syndrome-coronavirus) respiratory infections.
Respiratory hygiene/cough etiquette strategies designed to facilitate the prompt recognition of respiratory illness in patients and caregivers have been developed with the intention of incorporating these into infection prevention standard precautions (Table 11.5) [43]. Transmission-based precautions should be implemented for hospitalized patients with any signs or symptoms of respiratory viral infections pending diagnostic tests. A single patient room with toilet and hand hygiene facilities is preferred. If this is not feasible, spatial separation of >3 ft and the use of curtains or other room dividers are recommended. Other strategies, such as cohorting patients with the same organism or the same symptoms or cohorting providers, should be considered in outbreak or other special circumstances, in consultation with infection preventionists. When patients are transferred to other facilities or departments, the presence of a potentially communicable disease and current infection prevention precautions should be communicated to the receiving providers. Viral shedding may persist for weeks to months in immunocompromised patients. Discontinuing transmission-based precautions in this population, therefore, must consider host factors, disease epidemiology, and the results of diagnostic tests.
Table 11.5
Respiratory hygiene and cough etiquette components
1. Education of staff, patients, and visitors regarding respiratory hygiene and cough etiquette |
2. Posted signs instructing patients and caregivers to make healthcare personnel aware of symptoms of respiratory illness |
3. Provision of materials for source control (e.g., tissues, alcohol-based hand rub or supplies for handwashing after contact with respiratory secretions) |
4. Masking and spatial separation of persons with respiratory symptoms from others in common waiting areas (ideally in a single room, a minimum of 3 ft from others) |
5. Observance of droplet and standard precautions by healthcare providers when examining patients with symptoms of a respiratory infection |
Few high-quality randomized clinical trials have addressed the effectiveness of masks for the prevention of transmission of respiratory viruses. Existing evidence suggests, however, that both medical masks and respirators are effective. The CDC and other agencies recommend the use of these devices to protect patients and healthcare providers against seasonal influenza and tuberculosis. Clinical trials suggest that face masks provide compliance-dependent protection against infection in the community [44]. For healthcare providers, respirators appear to provide superior protection, but the choice of respiratory protection should be based on availability, etiology of illness, comfort, and the degree of risk.
Healthcare providers should refrain from working when ill with symptoms of a communicable respiratory infection; management policies should support and not discourage this practice. Likewise, visitors with respiratory illnesses should be discouraged from entering healthcare facilities unless this is unavoidable.
Seasonal influenza vaccination of healthcare providers reduces hospital-acquired influenza infections in cancer patients, and most evidence suggests that this practice decreases employee morbidity and absenteeism [45–47]. The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) recommends that, unless medical contraindications exist, all healthcare personnel should be vaccinated annually to protect themselves, their families, and their patients against influenza [48]. Similar recommendations exist for the use of tetanus-diphtheria-acellular pertussis (Tdap) vaccine for the prevention of pertussis [49]. These recommendations direct organizations to provide vaccine as part of employee health programs and to make efforts to reduce administrative and financial barriers to immunization [50]. Personnel refusing influenza vaccination for reasons other than a documented medical contraindication should sign a written declination that outlines the risks of vaccine refusal. Influenza immunization of HCP is tracked by the NHSN. During the 2014–2015 season, 88.6% of employees and 84.5% of all healthcare personnel in acute care hospitals received seasonal influenza vaccination, representing a significant increase over historical rates of compliance with vaccine recommendations [51, 52]. Successful strategies to improve compliance with vaccination policies have included education and incentive-based (i.e., reward) systems, but the most effective approaches have been mandatory vaccination policies that require the use of protective face masks or antiviral prophylaxis for the duration of the influenza season or result in the suspension or termination of unvaccinated workers [53]. Some states have enacted legislation to increase healthcare provider immunization rates [54].
Measurement
Both process (important data elements related to patient care activities) and outcome data must be systematically collected to prevent HAI. Ongoing process and outcome data collection can inform the development of more effective prevention strategies or lead to the modification of suboptimal processes. The latest CDC surveillance definitions should be used to identify the occurrence and rates of HAI within an institution [6]. If ongoing surveillance reveals a sharp increase in infections, standard epidemiological investigation techniques must be used to investigate the outbreak; these methods are beyond the scope of this chapter [55]. Organizations should remain open to reevaluating and improving measures for HAI based on new knowledge. For example, the recently developed definition for MBI-LCBI, clearly differentiating these infections from other CLABSI, is a relatively new outcome measurement that has substantial importance to the pediatric hematology-oncology population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree