Fig. 17.1
QOL assessments. Patients responded three times: (1) before each treatment, (2) 1 week after the first dose of cisplatin, and (3) at the end of the second course. (a) Score changes of global health status/QOL (items 29 and 30) in the EORTC QLQ-C30. (b) Score changes in the EORTC QLQ-LC 13 (Modified from Ref. [5])
Iressa Pan-Asia Study (IPASS) had also evaluated QOL [6]. QOL was assessed with the use of the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire and the Trial Outcome Index (TOI), which is the sum of the physical well-being, functional well-being, and Lung Cancer Subscale (LCS) scores of FACT-L, and symptoms were assessed with the use of the LCS score. QOL was significantly favored gefitinib in patients with EGFR mutation. The results were vice-versa in patients with EGFR mutation negative.
NEJ 002 study confirmed QOL advantage of gefitinib in patients with EGFR mutation positive [7]. QOL data from 148 patients (72 in the gefitinib arm and 76 in the carboplatin plus paclitaxel arm) were analyzed. Time to defined deterioration in physical and life well-being significantly favored gefitinib over chemotherapy.
LUX-Lung 3 study demonstrated PRO data favored afatinib compared to cisplatin plus pemetrexed in patients with EGFR mutation positive NSCLC [8]. Lung cancer symptoms and health-related QOL were assessed using the EORTC QLQ-C30 and EORTC QLQ-LC 13 questionnaires. Analyses of cough, dyspnea, and pain were preplanned, including percentage of patients who improved on therapy, time to deterioration of symptoms, and change in symptoms over time. First-line afatinib was associated with better control of cough and dyspnea compared with chemotherapy, although diarrhea, dysphagia, and sore mouth were worse. Global health status/QOL was also improved over time with afatinib compared with chemotherapy. These data indicate that molecular targeted therapy significantly improves QOL of the patients who have the driver oncogenes.
QOL data has been evaluated as a secondary endpoint so far. However, some recent studies use quality of life as the primary endpoint. ERACLE study was a randomized trial which compared cisplatin plus pemetrexed to carboplatin, paclitaxel plus bevacizumab [9]. The primary endpoint was the difference in QOL between the 2 treatment arms after 12 weeks of maintenance, measured using the EuroQoL 5 Dimensions-Index (EQ5D-I) and EQ5D-visual analogue scale (EQ5D-VAS). Although there was no difference between the two arms in EQ5D-visual analogue scale, EQ5D-I favored for cisplatin plus pemetrexed.
17.3 Palliative Care in Patients with Advanced Lung Cancer
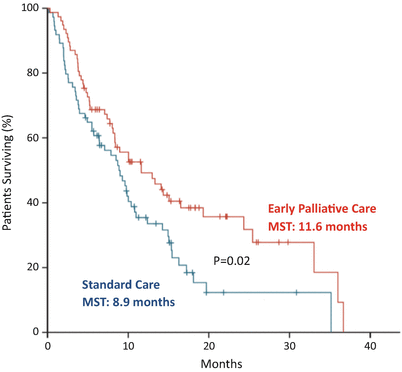
Temel JS and colleagues conducted a famous randomized trial of early palliative care (EPC) integrated with standard oncology care compared to standard oncologic care in patients with metastatic NSCLC [10]. The primary endpoint of the study was QOL at 12 weeks after randomization. This study revealed that EPC significantly improved QOL. Quality of terminal care was also better in EPC group with lower depression. Median OS was significantly longer in EPC group (11.6 months vs. 8.9 months, p = 0.02). Palliative care team conducted physical and psychological symptom control, establishing the goal of care and assisting decision making of patients. Furthermore, patients in EPC group had more accurate perception about prognosis compared to those in standard care group [11]. Patients in EPC group with accurate perception received less cytotoxic chemotherapy within 60 days before death than patients in standard care group. These data indicate that patients in EPC group could make more appropriate decisions (Fig. 17.2).
Another randomized trial results of early integration of palliative care with standard oncologic care versus late (3 months later) was reported [12]. Early-entry participants’ PROs and resource use were not statistically different. However, their survival 1 year after enrollment was improved compared with those who began 3 months later.
EPS studies indicate that early symptom management with psychosocial support and early illness understanding and assisting with decision-making of the patients should be considered as evidence-based patient management.
17.4 Communication Skill Training
To improve patient management, effective communication is critical. Based on Japanese patients’ preferences regarding breaking bad news, communication skill training program named SHARE protocol has been developed. SHARE consists of four components, including setting up supportive environment for interview, considering how to deliver the bad news, discussing additional information that patient would like to know, and providing reassurance and addressing patient’s emotions with empathic responses. SHARE protocol emphasizes reassurance and emotional support based on patients’ preference. Using the SHARE protocol, a randomized trial of communication skill training (CST) in oncologists who worked for National Cancer Center in Japan was conducted [13]. Oncologists were randomized to CST or control group. Both groups were evaluated pre- and post-CST by experts and themselves. Their patients were also evaluated regarding depression, anxiety, satisfaction with communication, and trust in oncologists. CST program consists of an hour lecture, 30 minutes of demonstration video, and an hour of role-play with simulated patients, eight times, in total 10 h and 2 days schedule. Thirty oncologists participated in the study. Backgrounds of the oncologists were similar in terms of age, clinical experience, gender, and specialty between the two groups. Performance of physicians was significantly improved by self-evaluation and experts’ evaluation. In total, 601 patients were evaluated. Age and gender were similar between the two groups. More patients in CST group were treated at surgical oncology and on current treatment. The HADS is a self-administered and standardized instrument for evaluating patients’ distress. HADS depression and trust in oncologist were significantly favored in patients who were treated by oncologists in CST group. Interestingly, time of consultation was not different in doctors after CST group. This is the first study that demonstrated improvement of patients’ outcomes by CST. CST program based on patient preference is effective for both oncologists and patients with cancer (Table 17.1).
Table 17.1




SHARE model for communication skill training
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree