17.3 Systems Approaches to Defining and Achieving Quality in Health Care
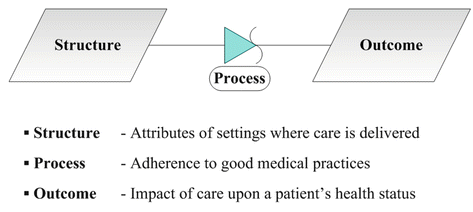
Through the development of the structure-process-outcome paradigm, Avedis Donabedian (1966, 1980, 1982, 1985) emphasized the importance of broad, systems-based approaches when considering quality improvement in health care (Fig. 17.3) [12–16]. Within this paradigm, it was advocated that initiatives to improve care consider strategies that incorporated aspects of structure (e.g., attributes of various delivery settings), process (e.g., employing sound medical practices), and outcomes (e.g., the impact of interventions on a patient’s health status). In more detail, structure involves attributes of an at-risk population, components of the system of healthcare delivery system (e.g., financing, organization, and availability of products or services), and the diverse physical, social, or economic environments surrounding patients, caregivers, and clinicians [12–16]. Process encompasses adherence to standards of care and involves an individual patient’s health risks (e.g., behavioral, environmental, genetic) and the specific transactions that are involved in obtaining care (e.g., utilization, satisfaction). Outcomes are those relevant health status measures at a patient or population level that extend beyond morbidity and mortality to include effectiveness, efficiency, or equity. Over time, seven pillars of quality of care emerged from Donabedian’s work: efficacy; optimality; acceptability; legitimacy; equity; cost; and efficiency [12–16] (Fig. 17.2).
To address more current and persistent quality deficiencies, the IOM built upon Donabedian’s work within the report Crossing the Quality Chasm to provide recommendations for safe, effective, patient-centered, timely, efficient, and equitable health care through a comprehensive system redesign (Figs. 17.3 and 17.4) [17]. Acknowledging the translational aspects that were also required to bridge experimental scientific work to that of real-world applicability, the IOM also defined quality of care as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.” [17]. As such, this definition captures prevention and treatment of conditions to involve those outcomes desired by patients and other stakeholders, also incorporating changing and evolving standards of care. Expanding upon this, the IOM also emphasized three broad criteria through which structure quality improvement prioritizes: (1) impact; (2) improvability; and (3) inclusiveness (Fig. 17.5) [18]. Therein, the aspects of healthcare delivery that were noted to potentially impact quality included resources or capacities of facilities (e.g., volume of services, scope of services, access to technology, staffing levels, academic affiliation), characteristics of healthcare providers and systems (e.g., level of training, specialization), and the financing, organization, and delivery of care (e.g., managed care, insurance mechanisms, regionalization of services) [18]. Additionally, it was recognized that a paucity existed in both the amount and strength of research relating to the structure-process-outcome paradigm in cancer. Although this issue persists, previously conducted research has reported associations between outcomes and structural components such as volume of cases, specialization, and provision of care [18].



Fig. 17.3
Six Aims Targeted by the Institute of Medicine (IOM) to Improve Health Care Systems, 2001 (Adapted from Source: Institute of Medicine [17])

Fig. 17.4
Three Criteria Used by the Institute of Medicine (IOM) to Identify Priority Areas for Improvement, 2003 (Adapted from Source: Institute of Medicine [18])
The overall systems redesign framework offered by the IOM provided specific recommendations concerning prevention, acute care, chronic treatment, and palliative care in addition to supporting patient-centered quality improvement goals [18]. Summarily, the IOM’s National Cancer Policy Board (1999) also recognized that optimal care may be compromised due to a patient’s social and economic status, belief system, provider decision-making, or lack of healthcare coverage [4]. Internationally, three goals established by the World Health Organization for healthcare systems emphasized that: (1) health status across populations be maximized across the spectrum of one’s life, to include end-of-life issues; (2) respectful treatment and orientation be addressed for patients, particularly concerning individual needs or preferences; and (3) financial protection be ensured by considering value propositions based upon an individual’s ability to pay [19].
17.4 Health Outcomes and Comparative Effectiveness Research
Outcomes research broadly encompasses the measurement and assessment of the impact of medical conditions and interventions upon the health status of patients [20–22]. The Agency for Healthcare Research and Quality (AHRQ) emphasized:
Outcomes research seeks to understand the end results of particular health care practices and interventions… End results include effects that people experience and care about, such as change in the ability to function. In particular, for individuals with chronic conditions – where cure is not always possible – end results include quality of life as well as mortality. By linking the care people get to the outcomes they experience, outcomes research has become the key to developing better ways to monitor and improve the quality of care. [20–22]
The National Cancer Institute (NCI) addressed outcomes research as a discipline which “describes, interprets, and predicts the impact of various influences, especially (but not exclusively) interventions, on ‘final’ endpoints that matter to decision makers” [21, 23].
Lipscomb et al. (2004) emphasized three requirements necessary for outcomes research to enhance the delivery of care: (1) developing valid and reliable outcome measures that were relevant for actual decision making; (2) providing supportive evidence that linked interventions to outcomes; and (3) developing an infrastructure necessary to translate empirical findings into pragmatic tools for decision makers [21, 24].
Presented in Fig. 17.5, a framework termed the ECHO model is often employed to encompass patient-centered economic, clinical, or humanistic outcomes [25]. Within this, economic outcomes involve the direct and indirect benefits attributed to a change in health state associated with treatment alternatives. Clinical outcomes involve those health-related events that occur subsequent to a medical condition or treatment intervention (i.e., morbidity or mortality), further classified as surrogate or intermediary endpoints (i.e., proxies of a person’s health status) or final endpoints (e.g., cure, treatment success, progression-free survival, death). Finally, humanistic outcomes involve those factors potentially impacting opinions about the consequences of a medical condition or intervention upon a person’s life or well-being and include utility, preferences, or health-related quality of life (HR-QoL), and patient-reported outcomes (PROs). Cost inputs to the ECHO model are those based upon varying perspectives of various decision makers or payers (i.e., “costs from the perspective of whom?”), consisting of direct medical costs associated with the direct medical treatment of a given disease state, direct nonmedical costs associated with the ability to receive medical care including transportation or lodging, and indirect costs involving lost productivity including time off work [26]. Treatment modifiers are comprised of various factors that may influence the various outcomes of interest including, for example, adverse events or nonadherence. Notably, the ECHO framework often involves the simultaneous assessment of multiple types of outcomes that are often disease related.
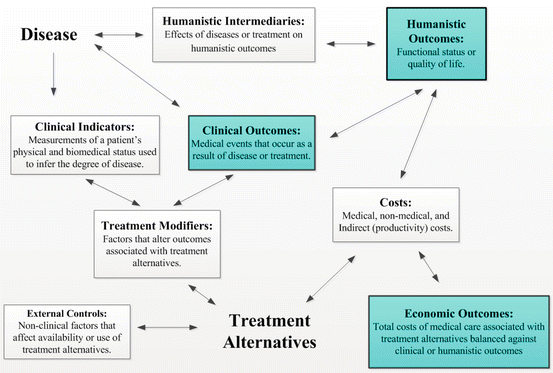

Fig. 17.5
The Economic, Clinical, and Humanistic Outcomes (ECHO) Framework (Source: Kozma et al. [25])
An additional and important consideration within the ECHO model involves the distinct differences between surrogate endpoints or intermediaries versus final outcomes or end results, in particular, because several clinical intermediaries (i.e., results of laboratory tests) might only partially correlate to clinical morbidity of mortality outcomes or other outcomes that might be of key importance to patients themselves (e.g., quality of life, preferences, utilities) [21, 25, 26]. In a broad sense, also applied to goals of a comprehensive health system, several difficulties exist in ranking the various importance of either the types of final outcomes (e.g., economic, clinical, humanistic) or their corresponding intermediaries in a rank-order fashion to measure quality of care; Evans et al. confronted this issue by emphasizing that a key intrinsic goal of any health system simply remains one whose attainment is desirable in itself, irrespective of all others [27].
Specific to oncology, Lipscomb et al. (2004) emphasized that final outcomes are typically clinically oriented (e.g., overall survival, disease/progression-free survival, or relapse) though also embody humanistic measures (e.g., HR-QoL, perceptions of or satisfaction with care, patient preferences, economic utility) and economic outcomes (i.e., a monetized value of health status changes) [21]. Extending clinical outcomes to incorporate measures of both morbidity and mortality, the quality-adjusted life year (QALY) incorporates both survival with a measurement or estimation of economic utilities that reflect a patient’s quality of life or preferences [28]. Further, Tate and Skrepnek (2014) also discussed the application of quality-adjusted time without symptoms or toxicity (Q-TWiST), an additional patient-reported outcome that adjusts survival measures with changes in quality of life [29]. Pursuant to an informal IOM survey on value assessments in cancer, Fig. 17.6 presents the diversity of conceptions and metrics that may be present [7].
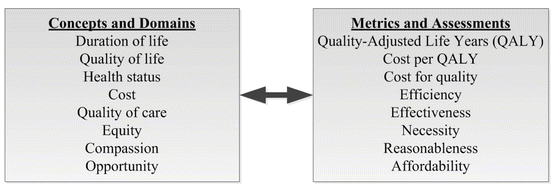

Fig. 17.6
Attributes of Value Identified via an Informal Workshop Survey at the Institute of Medicine (IOM), 2009 (Adapted from source: Institute of Medicine [33])
Surrogate outcomes in cancer may include relevant laboratory data (e.g., hematologic or molecular responses) or disease changes (e.g., tumor shrinkage or disappearance), though other intermediate endpoints might involve screenings, smoking cessation, or the use of adjuvant therapies and various treatment protocols [21, 26]. While acknowledging these surrogate measures is crucial in assessing the quality of patient care, a need is also present to understand and quantify correlates between intermediaries and final outcomes. As noted by Lipscomb et al. (2004) and Skrepnek (2005), a key corollary of an observed improvement in either clinical or intermediate outcomes is that of prediction of success in improving end results of care [21, 26].
Given that the majority of outcomes research in cancer tends to focus upon screening, diagnosis, and treatment interventions, it is emphasized that its contributions (particularly the emerging roles of PROs) cross the continuum from prevention and early detection to survivorship and end-of-life care [7, 21, 30]. While more consensus exists surrounding the definition and measurement of final outcomes, despite notable exceptions (e.g., defining disease- or cause-specific versus relative survival), considerable discussion surrounds the appropriate assessment particularly of humanistic outcomes including utility, PROs, and satisfaction [21].
A central tenet of the 2010 Patient Protection and Affordable Care Act (ACA), comparative effectiveness research (CER) emerged as an approach to increase the efficiency and amount of comprehensive evidence-based information available to inform patients and providers within the US healthcare system [30, 31]. Several methodological approaches inherently comprise CER, though the general empirical premise emphasizes real-world investigations of representative populations, personalized interventions, patient-centered outcomes, and active treatment comparators as opposed to placebo controls. More formally addressed by the IOM, CER involves the “generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at both the individual and population levels” [32, 33].
Clinical evidence within CER is categorized as either primary or secondary. Primary clinical evidence involves the direct reporting of new empirical findings through experimental or quasi-experimental studies to include a range of clinical trials (e.g., pragmatic, cluster randomized, large simple clinical trials) or observational studies (e.g., retrospective case-control or cohort studies) [31–33]. While prospective data collection may be involved, existing data sources (e.g., electronic medical records, administrative claims data, registries) are also considered. Secondary clinical evidence in CER involves the systematic collection and synthesis of primary research findings, essentially pooling results from multiple investigations to support conclusions with improved statistical power (e.g., meta-analyses, decision-analytic modeling, mixed treatment comparators) [31–34].
Despite the recognition that the Patient-Centered Outcomes Research Institute (PCORI) was formalized partially in response to increasing healthcare expenditures, CER explicitly omits assessments of costs (either by way of resources consumed or in terms of a monetized change in health status) as defined within the ACA [30]. The challenges and controversies associated with health state valuations and concerns of rationing have emphasized the role of CER as providing outcomes evidence that may be used within other health economic or cost-effectiveness investigations [26, 28, 30].
17.5 Comprehensive Economic Evaluations: Cost-Effectiveness, Cost-Utility, and Cost-Benefit
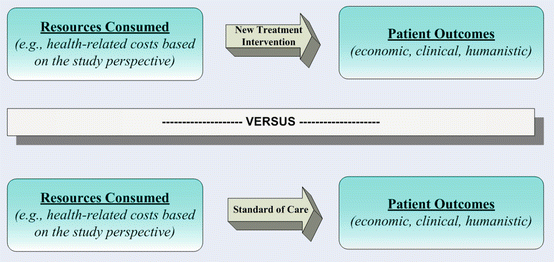
Despite the intuitive rationale to study outcomes to improve quality of care in cancer, measuring economic, clinical, or humanistic outcomes alone does not afford an opportunity to assess or specify which interventions might most efficiently improve care across diverse populations or circumstances [26, 35]. A complete framework to evaluate the value of innovative treatment interventions in terms would seek to assess both resources consumed and outcomes achieved of novel approaches versus appropriate clinical alternatives, illustrated in Fig. 17.7 [10, 26, 36].
Expanding the scope of CER to include both costs and outcomes of alternative treatments, health technology assessments and pharmacoeconomic analyses are inherently based upon principals of cost-benefit analyses, welfare economics, and game theory [10, 26, 36]. Applied specifically to the use of pharmaceutical services or products, pharmacoeconomics is characterized by the comprehensive identification, measurement, and comparison of healthcare resources consumed and various patient outcomes for novel interventions compared to existing standards of care [26, 36]. Given that a principal goal of these analyses is to yield the greatest patient benefit in terms of outcomes for every healthcare dollar expended, the formal framework identifies opportunity costs gained or lost and, therefore, provides decision makers a theoretically sound approach to address the role of emerging healthcare innovation within the health policy triad of cost, quality, and access [10, 26, 36]. Across the continuum of cancer, value propositions ranging from prevention to survivorship may consider numerous specific stakeholders (e.g., providers, payers, patients), or society overall. Particularly from the societal perspective, the most effective, efficient, and equitable use of innovation would be afforded via an optimal balance of costs and benefits to afford the greatest net benefit across an entire population [26]. Comprehensive assessments of costs and outcomes across treatment alternatives uniquely augment evidence-based decision making when considering pressures of cost-containment, resource scarcity, increased expenditures, and treatment advances [10, 26].
Essentially, this methodological approach may provide a more robust assessment of quality and to augment value-based competition when considering expanding treatment options and increasing healthcare utilization and cost. Therein, value propositions of healthcare innovations are deemed “cost-effective” under three scenarios, when the novel approach is either: (1) less expensive and at least as effective as existing standards of care; (2) less expensive and less effective, though only if an extra benefit is not worth an extra expense; or (3) more expensive and more effective under conditions that the additional health outcome benefit is worth the additional cost, up to a certain opportunity cost threshold value [26, 28].
Overall, the various types of studies that comprise health technology assessments or pharmacoeconomics are presented in Fig. 17.8. Notably, full economic analyses are designated with cost-effectiveness, cost-utility, or cost-benefit analyses and may be best characterized by their application of incremental calculus and subsequent measurement of opportunity costs to compare both costs and outcomes for new interventions versus existing standards of care.
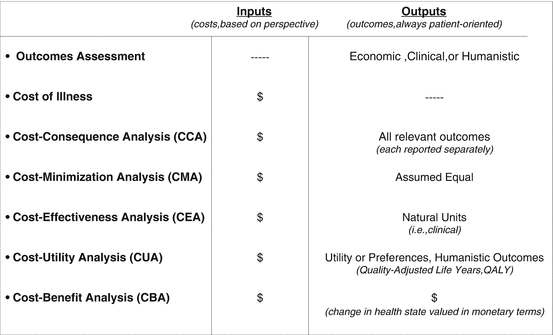

Fig. 17.8
Selected Types of Health Technology Assessment or Pharmacoeconomic Study Designs
Addressed previously, even though economic analyses provide information to maximize patient outcomes when resources are limited, ethics or morals may be not be comprehensively captured within all specific types of assessments conducted. As Dougherty (1993) stated, “an ethical imperative to save individual lives even when money might be more efficiently spent to prevent deaths in the larger population” is often characteristic of the types of interventions offered within rare, progressive, or terminal diseases, more formally called the rule of rescue [28, 37]. Continued assessments of both ethical and economic issues that are involved in the adoption and reimbursement of novel interventions are warranted across rare, progressive, and terminal conditions in light of new advances in screening or diagnostic procedures and personalized or targeted therapeutics [28].
17.5.1 Burden or Cost of Illness (BoI, CoI): Budget Impact Analysis (BIA)
Burden or Cost of Illness (BoI, CoI) analyses predominantly seek to identify costs consumed (e.g., those associated with treatment), but may extend to include work loss, morbidity, and mortality [38–41]. Therefore, outcomes may be measured but are usually not formally analyzed with costs. Overall, these investigations may be useful to bring awareness to the impact that a given disease or health state may have upon patients, payers, or society to assist decision makers to allocate appropriate budgets, to estimate payment structures, or to better understand trends in care. BoI/CoI studies that focus on long-term costs at the patient level may additionally provide key inputs to other economic studies. Presented in Fig. 17.9, Lipscomb et al. (2004) offered a conceptual framework to cost cancer-related healthcare episodes that spans precancer cases through end-of-life care to organ acquisition or donation [42].
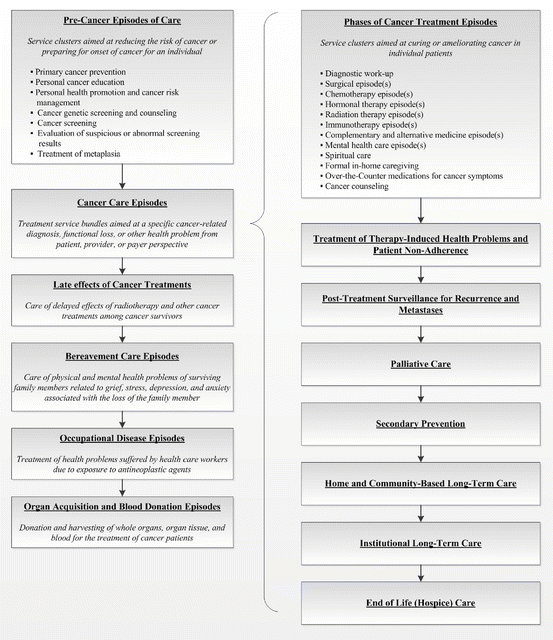

Fig. 17.9
A Conceptual Framework for Costing Cancer-Related Health Care Episodes (Source: Lipscomb et al. [42])
The categorization of BoI or CoI studies involves two parts: (1) prevalence versus incidence models and (2) excess versus attributable costing [39, 40]. Prevalence models are cross-sectional in nature, reflecting costs in a given time period (e.g., annual) and comprising the most common approach. Conversely, incidence models involve lifetime costs (i.e., spanning from the onset of disease to cure or death), typically requiring estimation of future costs associated with a given disease. In excess costing, a case-control methodology is used wherein those with a specified condition are matched or compared against a control group without the disease, or simply compared to an overall or available population. Thus, results reflect the additional impact that occurs as a result of having a given condition or disease. The attributable costing method uses a case-series approach where a burden of illness is measured only among those with a given disease. While results seek to report overall resource utilization and costs associated with any given disease state, the attributed BoI study may separately report selected subgroups or comorbid conditions, essentially borrowing elements of excess costing.
A pragmatic approach used to apply findings, in part, from BoI/CoI studies may include a budget impact analysis (BIA) [43, 44]. BIAs seek to measure the net cost of treatment associated for any given number of type of patient within specific healthcare settings or patient population. Historically, although BIAs often focus on specific healthcare services or pharmacy expenses, comprehensive direct medical costs may be incorporated by implementing broad comparative cost determinations across competing scenarios (e.g., single or multiple therapeutic areas or diseases).
17.5.2 Cost-Consequence Analysis (CCA)
Cost-consequence analyses (CCA) measure all relevant costs and outcomes, though only list values without formally incorporating outcomes in a comprehensive fashion [45, 46]. Therefore, responsibility is placed upon the decision maker to analyze data and draw comparisons of efficiencies associated with any given innovation relative to any standard of care. While the CCA may promote transparency within full economic analyses, in themselves these investigations are rarely stand-alone given concerns that inappropriate conclusions may be drawn concerning the information presented.
17.5.3 Cost-Minimization Analysis (CMA)
A cost-minimization analysis (CMA) assesses differences in costs (measured in dollars and based upon the perspective of a study’s selected payer of an innovation versus a reference standard of care) while assuming that patient outcomes or health benefits are equivalent [45, 46]. Given this, the approach is a straightforward concern of assessing costs, though requiring that the equivalency of outcomes be firmly established (e.g., via clinical trials, particularly those designed to establish equivalence). Though intuitive in its interpretation (e.g., dollars saved), controversy does surround a definition of equivalency in outcomes, particularly if the measurement of an economic, clinical, or humanistic outcome may potentially differ across treatment options, or if equivalence is derived via subjective means [46]. Further, clinical efficacy measured from experimental, randomized clinical trials may poorly translate to real-world effectiveness in which the CMA often seeks to address.
Given that the objective of the CMA is to assess costs and not to compare health outcomes, researchers may not necessarily designate the method as a full economic analysis [47]. Even though the approach may remain a conceptually weaker form of pharmacoeconomic evaluation, the strength of results emanating from a CMA center upon the strength of evidence that outcomes are indeed the same – a presumption that may be inadequately addressed especially with regard to humanistic outcomes including preferences.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree