1. Diameter >1 cm (or in the case of the jugulodigastric nodes, >1.5 cm)
2. Smaller than 1 cm with spherical rather than ellipsoidal (or “kidney-bean”) shape
3. Contain inhomogeneities suggestive of necrotic centers
5. FDG-PET positive
4.3.2 Defining the Nodal Levels at Risk in the Neck
Much as the CTV surrounding the primary tumor encompasses tissue deemed to be at risk for microscopic tumor extension, the CTVs in the neck consist of the lymph node levels containing nodes not meeting radiologic criteria for gross involvement that are nonetheless at risk for microscopic involvement. The bulk of modern knowledge on the pattern of lymphatic drainage from each head and neck site is based upon the classic anatomical work by Rouvière, the descriptive report by Lindberg on the prevalence and distribution of clinical neck metastases at presentation, and reports on the incidence and distribution of microscopic pathological lymph node involvement at elective neck dissection by Byers et al., Candela et al., and others (Mukherji et al. 2001; Rouvière 1938; Candela et al. 1990; Lindberg 1972; Byers et al. 1988). Taken together, this body of literature demonstrates that squamous cell carcinomas of the upper aerodigestive tract tend to metastasize to the neck in predictable patterns, with the distribution and likelihood of involvement governed by the density and drainage of the lymphatics at each mucosal site, and with increasing risk at each nodal level if the adjoining proximal level is involved.
An anatomical reference standard for classifying neck lymph node regions was devised by surgeons from the Memorial Sloan Kettering Hospital and revised by Robbins et al. and Robbins (Robbins 1998; Robbins et al. 1991; Shah et al. 1981) (Fig. 4.1). This classification defined six neck levels with three sublevel divisions, each with discrete anatomical boundaries that can be readily identified during neck dissection to facilitate standardized reporting of the locations of nodal involvement and the extent of surgical therapy in the neck. A corresponding nodal level classification system based upon landmarks readily identifiable on axial imaging was subsequently proposed by Som et al. (1999). The application of this classification system toward CTV delineation in the neck has been extensively described and has been integrated into multinational cooperative group consensus panel guidelines, summarized in Table 4.2 (Gregoire et al. 2000; Gregoire and Levendag; Nowak et al. 1999; Wijers et al. 1999) and available online at http://www.rtog.org/corelab/contouringatlases/hn.aspx. Gregoire et al. recently published an extensive review of the literature on the risk of metastases to each neck level, with accompanying elective nodal coverage recommendations by primary tumor site (Gregoire et al. 2000). Eisbruch et al. have similarly proposed more detailed guidelines for target selection in the neck (Eisbruch et al. 2002). These publications and the DAHANCA, EORTC, GORTEC, NCIC, and RTOG consensus panel contouring guidelines and atlas are highly recommended for the reader before embarking upon three-dimensional conformal radiation therapy or IMRT for the treatment of head and neck cancer.
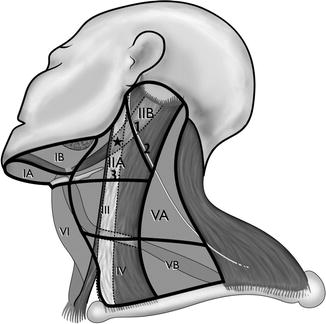

Fig. 4.1
Neck node levels. 1 Posterior belly of the digastric muscle; 2 accessory nerve; 3 jugular vein; asterisk jugulodigastric nodes: where jugular vein bisects the posterior belly of the digastric muscle (just below the transverse process of C1)
Table 4.2
Radiographic anatomical boundaries for lymph node levels in the clinically node negative and surgically unviolated neck
Cranial | Caudal | Anterior | Posterior | Lateral | Medial | |
|---|---|---|---|---|---|---|
IA Submental nodes | Geniohyoid and mylohyoid muscles | Central body of the hyoid bone | Symphysis menti and platysma muscle | Body of the hyoid bone | Medial aspect of the anterior belly of the digastric muscle | N/A (midline nodal region) |
IB Submandibular nodes | Mylohyoid muscle and cranial aspect of submandibular gland | Central body of the hyoid bone | Symphysis menti and platysma muscle | Posterior aspect of the submandibular gland | Medial aspect of the mandible, platysma muscle, or skin | Lateral aspect of the anterior belly of the digastric muscle |
IIA Upper jugular nodes (anterior) | Caudal aspect of lateral process of C1a | Caudal aspect of the body of the hyoid bone | Posterior aspect of the submandibular gland; anterior aspect of the ICA; posterior aspect of the posterior belly of the digastric muscle | Posterior border of the IJV | Medial aspect of the SCM | Medial aspect of the ICA; paraspinal muscles (levator scapulae and scalenes) |
IIB Upper jugular nodes (posterior) | Caudal aspect of lateral process of C1a | Caudal aspect of the body of the hyoid bone | Posterior border of the IJV | Posterior border of the SCM | Medial aspect of the SCM | Medial aspect of the ICA; paraspinal muscles (levator scapulae and scalenes) |
III Middle jugular nodes | Caudal aspect of the body of the hyoid bone | Caudal aspect of the cricoid cartilage | Posterolateral aspect of the sternohyoid muscles and anterior aspect of the SCM | Posterior aspect of the SCM | Medial aspect of the SCM | Medial aspect of the internal/common carotid artery; paraspinal muscles (scalenes) |
IV Lower jugular nodes | Caudal aspect of the cricoid cartilage | 2 cm cranial to the sternoclavicular joint | Anteromedial aspect of the SCM | Posterior aspect of the SCM | Medial aspect of the SCM | Medial aspect of the common carotid artery; paraspinal muscles (scalenes) |
VA Posterior triangle (upper) | Skull base | Caudal aspect of the cricoid cartilage | Posterior aspect of the SCM | Anterior border of the trapezius muscle | Platysma muscle and skin | Paraspinal muscles (levator scapulae, scalenes, splenius capitis) |
VB Posterior triangle/spinal accessory nodes (lower) | Caudal aspect of the cricoid cartilage | Transverse cervical vessels | Posterior aspect of the SCM | Anterior border of the trapezius muscle | Platysma muscle and skin | Paraspinal muscles (levator scapulae, scalenes, splenius capitis) |
VI Pretracheal and paratracheal nodes | Caudal aspect of body of the thyroid cartilage (pretracheal nodes) or cricoid cartilage (pretracheal nodes) | Sternal manubrium | Skin and platysma muscle | Posterior aspect of the trachea; anterior aspect of cricoid cartilage | Medial aspect of the internal/common carotid artery | N/A (midline nodal region) |
VII Upper mediastinal nodes | Sternal manubrium | Innominate vein | Posterior aspect of the sternal manubrium | Tracheoesophageal groove | Lateral aspect of the common carotid artery | N/A (midline nodal region) |
RP Retropharyngeal nodes | Base of the skull | Caudal aspect of the C2 vertebral body | Fascia underlying the pharyngeal mucosa | Prevertebral muscles (longus colli, longus capitis) | Medial aspect of the internal carotid artery | Midlineb |
In determining whether to include a specific nodal level in the CTV, we typically consider that a 10 % or higher risk of metastatic involvement warrants elective treatment, whereas a less than 10 % risk of microscopic involvement may be inadequate to justify the risk of complications from prophylactic RT. The factors that dictate this risk for each nodal level vary from case to case and depend on primary tumor site, tumor stage, tumor size, thickness or depth of invasion (i.e., 3 mm or more is associated with a high metastatic risk for oral cavity tumors), tumor grade/differentiation, keratinization status, lymphatic vessel invasion in the tumor specimen, and the presence, distribution, and extent of nodal involvement (Eisbruch et al. 2002). For tumors of the major or minor salivary glands, high-grade or large tumors harbor a significant risk of subclinical nodal involvement warranting prophylactic ipsilateral nodal coverage (or bilateral coverage for soft palate tumors), with the notable exception of adenoid cystic carcinoma and acinic cell carcinoma, which infrequently spread to lymph nodes (Armstrong et al. 1992; Chen et al. 2007b).
4.3.3 Specific Clinical Target Volumes in the Neck
In selecting the CTVs for head and neck cancer IMRT, the following nodal levels should be included where appropriate, as described. While these general concepts are broadly useful, specific approaches for individual tumor sites have been previously described elsewhere in detail (Eisbruch et al. 2002; Million 1994).
4.3.3.1 Level II Nodes
Level II is the most frequent nodal metastatic site for tumors in all mucosal sites. This level can be subdivided into the internal jugular (IIA) and spinal accessory (IIB) subdivisions. In the surgical classification system, the spinal accessory nerve serves as the division between these levels, with level IIA lying anteriorly and IIB posteriorly (Fig. 4.1) (Robbins 1998). Due to the difficulty in discerning the division between these levels on axial imaging, however, the adapted radiographic classification instead defined these nodes relative to the posterior border of the internal jugular vein, which can be readily visualized on a simulation CT scan (Som et al. 1999). By this widely adopted system, level IIA includes those nodes located anteriorly, laterally, or medially to the internal jugular vein as well as those located posteriorly without a fat plane separating them from the jugular vein; level IIB nodes lie posterior to jugular vein with an intervening fat plane, thus approximating a location posterior to the spinal accessory nerve. The important specific nodal stations within level II include the subdigastric (jugulodigastric) node, which is located within level IIA immediately inferior to the crossing of the posterior belly of the digastric muscle past the internal jugular vein, and the more cranially located “junctional” node, aptly named for its location within level IIB at the superior junction of the jugular nodal chain (level II) and spinal accessory nodal chain (level V) (Rouvière 1938). The subdigastric node is the most commonly involved node for both ipsilateral and contralateral nodal metastases from primary tumors of the oropharynx, supraglottic larynx, and hypopharynx, while the more cephalad junctional node is the first echelon node for the nasopharynx and parotid gland, which is commonly involved in cases of nasopharyngeal cancer and is at risk in the case of coexistent metastases to levels II, III, or VA (Million 1994). Therefore, the ipsilateral subdigastric node and level IIA below the transverse process of C1 should be included in the CTV for all N0 patients with cancers arising in most head and neck mucosal sites receiving definitive radiotherapy (with or without chemotherapy), with the notable exception of early stage, node-negative squamous cell carcinomas of the glottic larynx (i.e., T1–2N0). Ipsilateral level IIA superior to the C1 transverse process and level IIB, meanwhile, are included in the CTV in all patients with nasopharynx primary cancers, irrespective of nodal involvement, and in those patients with other primary sites with nodal metastases in the ipsilateral level II. Contralateral level IIA below the C1 transverse process should be included in the elective CTV in all cases of mucosal squamous cell carcinomas originating in sites with bilateral lymphatic drainage (i.e., base of the tongue, soft palate, nasopharynx, supraglottic larynx, hypopharynx) or in any case of N2a or greater nodal involvement with any primary mucosal tumor site (Fig. 4.2). In contrast, contralateral level IIA superior to the C1 transverse process and level IIB can be safely excluded from the CTV in non-nasopharyngeal cancer patients without involvement of the adjacent level II, thus allowing for sparing of the majority of the contralateral parotid gland and a significant preservation of salivary function (Eisbruch et al. 2001; Beetz et al. 2012) (Fig. 4.3).
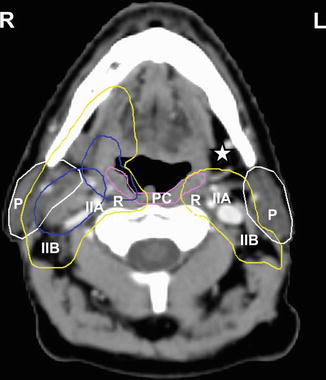
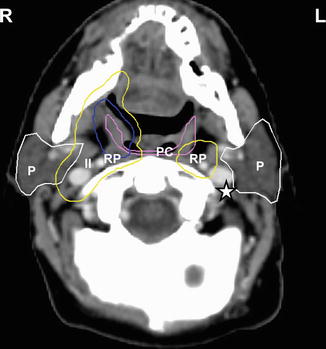

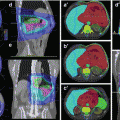
Fig. 4.2
Axial contrast-enhanced CT image of a patient with a right tonsillar T1 N2a M0 squamous cell carcinoma with involvement of the ipsilateral subdigastric node (level IIA). The PTV surrounding the gross primary and nodal disease is displayed in blue. At this level below the transverse process of C1, the bilateral level IIA and IIB (II) and the RP nodes are included in the subclinical PTV bilaterally (yellow). As demonstrated, right level IB located ipsilateral to the involved node and primary tumor is included in the subclinical PTV (yellow), while the contralateral left level IB is excluded (white asterisk). Important organs at risk contoured at this level include the tail of the parotid glands (P, white contour) and superior pharyngeal constrictor muscle (PC, violet)

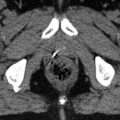
Fig. 4.3
Axial CT image C1 in the same patient as Fig. 4.2 (right tonsillar T1 N2a M0 squamous cell carcinoma). The primary tumor PTV in displayed in blue. At this axial level near the base of the skull superior to the transverse process of C1, left level II (star) in the uninvolved contralateral neck is excluded, while right level II (II) and the bilateral RP nodes (RP) are included in the subclinical PTV (yellow). The parotid glands (P, white contour) and superior pharyngeal constrictor muscle (PC, violet) are among the important structures to delineate for avoidance
4.3.3.2 Level III
Level III is included in the CTV when there is a risk of subclinical disease or if level II on the same side of the neck is grossly involved. Designation of level III as a high-risk or low-risk CTV is dependent on whether lymph nodes in this level are grossly involved (as described below).
4.3.3.3 Level IB Nodes
Level IB is treated in all cases with a significant clinical involvement of ipsilateral level II (e.g., oropharyngeal cancer with nodal involvement of level II of >N1) and in primary tumors that drain directly to level IB (i.e., oral tongue, floor of the mouth, anterior tonsillar pillar, retromolar trigone, buccal mucosa), irrespective of clinical nodal involvement (Figs. 4.3 and 4.4). Level IB should additionally be included for oropharyngeal cancers of the tonsil or base of the tongue with a significant anterior extension to the anterior tonsillar pillar or oral tongue, respectively. For well-lateralized primary tumors (i.e., buccal mucosa, retromolar trigone), only the ipsilateral side can be treated, whereas more midline tumors (i.e., floor of the mouth) or those with bilateral lymphatic drainage (i.e., oral tongue for both level IB) require bilateral coverage. Exclusion of level IB from the CTV in cases lacking the above risk factors facilitates partial sparing of the submandibular gland, preserving non-stimulated, mucin-rich submandibular salivary flow and thereby reducing xerostomia (Eisbruch et al. 2001; Little et al. 2012; Murdoch-Kinch et al. 2008).
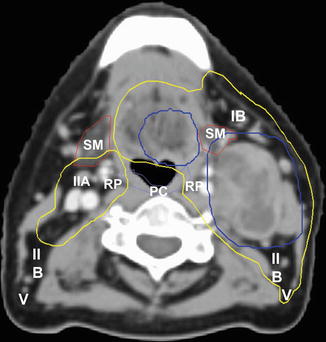

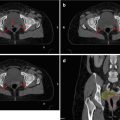
Fig. 4.4
Axial CT image of a patient with a left base of the tongue T1 N2a squamous cell carcinoma. PTVs for the primary tumor and involved left level II node are displayed in blue. Level IB and V on the ipsilateral left side are included in the elective PTV (yellow), whereas the contralateral right-sided elective PTV excludes level IB, posterior IIB, and V. The bilateral RP nodes (RP) are additionally included bilaterally. The superior pharyngeal constrictor muscles (PC, violet) and submandibular glands (SM, brown) are notable OARs at this level
4.3.3.4 Level IV Nodes
Level IV is treated in all cases of either ipsilateral level II or III involvement and in primary sites that drain directly to level IV, regardless of additional nodal involvement (i.e., tip of the oral tongue, supraglottic larynx, hypopharynx). As is the case for level IB above, midline tumors and those with bilateral lymphatic drainage (i.e., oral tongue, posterior pharyngeal wall) require bilateral level IV coverage.
4.3.3.5 Level V Nodes
Inclusion of level V in the CTV is advised in all cases of a significant (>N1) involvement of levels II–IV on the same side of the neck (Fig. 4.4) as well as in primary tumors of the nasopharynx, which drains directly to level V.
4.3.3.6 Retropharyngeal Nodes
The risk of RP involvement relates strongly to the primary tumor site and to the extent of jugular chain nodal involvement (McLaughlin et al. 1995). As such, the lateral retropharyngeal (RP) nodes are treated bilaterally in all cases of nasopharyngeal cancer, tumors that involve the posterior pharyngeal wall, and oropharyngeal and hypopharyngeal cancers with clinical involvement of levels II–IV (Figs. 4.2 and 4.3). At present, our policy is to limit the coverage of the lateral RP nodes to the ipsilateral side only in cases of early lateralized oropharyngeal tumors with N0 or small-volume N1 disease. Recent preliminary evidence, however, suggests that the contralateral RP nodes may be safely excluded from the CTV in oropharyngeal cancers without contralateral jugular chain involvement, which if supported by more mature and conclusive evidence will provide a strong rationale for limiting treatment to only the ipsilateral RP nodes in this circumstance as well (Spencer et al. 2012). The medial RP nodes are included only in tumors that involve the posterior pharyngeal wall.
4.3.3.7 Level VI Nodes
Level VI nodes are treated in all cases with clinical involvement of level IV nodes, in cases of hypopharynx cancer where the apex of the pyriform sinus is involved, laryngeal cancers extending to the subglottic larynx, and in all cases of thyroid cancer for which RT is indicated.
4.3.3.8 Level VII Nodes
Level VII nodes are treated in all cases of gross level VI nodal involvement.
4.3.4 Special Considerations for Target Delineation
A number of aforementioned anatomical and surgical studies form the fundamental basis for elective nodal CTV coverage in squamous cell carcinoma of the head and neck (Byers et al. 1988; Candela et al. 1990; Lindberg 1972; Rouvière 1938). Further rationale for these CTV definition guidelines when using IMRT is provided by patterns of failure studies from the University of Michigan and other institutions (Chen et al. 2010, 2011; Dawson et al. 2000; Eisbruch et al. 2004a). One series of 133 patients from our institution with non-nasopharyngeal HNC treated with either definitive or postoperative IMRT with sparing of the parotid glands evaluated the site of failure in each of 21 patients with locoregional failure (Dawson et al. 2000; Eisbruch et al. 2004a). All patients in this study, even those who were clinically node negative, received bilateral neck irradiation due to a high risk of subclinical bilateral neck disease. The superior borders of the CTV in most patients was the skull base for the ipsilateral neck, the level of the posterior belly of the digastric muscle crossing the internal jugular vein for the contralateral neck, and the top of the C1 vertebral body for the RP nodes, in accordance with Rouvière’s description of the location of the lateral RP nodes (Figs. 4.2 and 4.3) (Rouvière 1938). At a median follow-up period of 32 months, 21 of the 133 patients (16 %) had locoregional recurrences; 17 occurred infield, whereas four were marginal. The findings from this study and other institutions underscore the following particular concerns with the use of IMRT for head and neck cancer.
4.3.4.1 Retropharyngeal Nodes
Two of the four marginal recurrences in our institutional series occurred near the base of the skull at the site of the lateral RP nodes (Eisbruch et al. 2004a). Both occurred in patients with oropharyngeal cancer (one tonsillar and one base of the tongue) who presented with N0 necks, in whom the RP nodes had been included within the CTV with a cranial most extent to the top of C1. However, the epicenter of the recurrence volume of the two marginal RP recurrences lays cranial to the top of C1, one ipsilateral to the primary tumor and the other contralateral and extensive. Given this unexpected pattern of failure, and the scant details in the literature regarding the pattern of metastases to the RP nodes in non-nasopharyngeal cancers, our institutional policy was modified to include the lateral RP nodes bilaterally with a cranial extent to the skull base in all cases of locally advanced oropharyngeal cancer, irrespective of extent of neck node involvement (Figs. 4.2 and 4.3) (King et al. 2000; McLaughlin et al. 1995). This practice is consistent with the recent cooperative group consensus guidelines for CTV delineation and is supported by surgical series of RP node involvement, other institutional case reports of marginal RP failures near the skull base, and RT series demonstrating an absence of RP nodal failures following IMRT using elective CTVs extending to the skull base (Chen et al. 2011; Chung et al. 2011; Coskun et al. 2011; Eisbruch et al. 2004a; Gregoire and Levendag; Hasegawa and Matsuura 1994). One recent preliminary report, however, suggested that in selected cases of ipsilateral-only nodal involvement, treatment of the contralateral RP nodes can be avoided without an increased risk of marginal or out-of-field failure (Spencer et al. 2012). If supported by more mature and conclusive evidence, exclusion of the contralateral RP nodes in cases of locally advanced oropharynx cancer without contralateral nodal involvement will be incorporated into our future treatment policies and recommendations.
4.3.4.2 Lymph Nodes at Risk Due to Aberrant Lymphatic Drainage from Extensive Neck Disease
The third marginal recurrence in our institutional series was in a patient with oral tongue cancer who had bulky low neck nodal involvement (Eisbruch et al. 2004a). Despite achieving a complete response after RT, the patient subsequently recurred in level VI, highlighting the potential for involvement retrograde lymphatic flow and unpredictable patterns of nodal metastasis in patients with bulky nodes (Fisch 1968). Rouvière described lymphatic drainage channels to the pretracheal/prelaryngeal nodes (level VI) from the jugular nodes (levels III–IV) which provide a conduit for distal lymphatic drainage in cases of extensive nodal involvement (Rouvière 1938). These connections, in tandem with flow obstruction at level IV, explain this marginal recurrence at level VI. As such, level VI nodes should be treated in all cases with clinical involvement of level IV nodes.
A recent review of our institutional experience between 2003 and 2011 revealed six cases of patients with primary cancers of the oropharynx and nasopharynx who presented with pathologically confirmed parotidean metastases (unpublished data). All of these patients had locally advanced stage N2c or N3 disease, with diffuse multilevel and bulky involvement of ipsilateral level II. Based on these findings, we have changed out institutional practice to include elective coverage of the ipsilateral parotidean nodes in all cases of extensive involvement of the level II lymph nodes.
4.3.4.3 Prior Surgery
The fourth marginal recurrence in our institutional patterns of failure series was observed in a patient who had a history of prior neck dissection more than 1 year prior to recurrence of an oral cavity cancer (Eisbruch et al. 2004a). He was treated with local excision of the primary site recurrence followed by postoperative IMRT and experienced subsequent recurrence in the subcutaneous tissues of contralateral level I – an unpredictable location given the site of his primary tumor. As in patients with extensive nodal involvement, those patients with a history of neck surgery more than 1 year prior to tumor recurrence may experience unpredictable lymphatic drainage, in this case due to collateral lymphatics which fully develop 1 or more years after surgery (Fisch 1968; Rouvière 1938). These collaterals commonly develop in the submental and submandibular areas, including within the subdermal tissues, thus leading to unpredictable patterns of recurrence. As such, the use of IMRT in patients who have had major neck surgery more than 1 year prior to tumor diagnosis should be approached with caution due to the uncertainty regarding their targets.
4.3.4.4 Selection of Split-Neck Versus Whole-Neck IMRT for Supraclavicular Treatment
Chao et al. reported the outcomes of 126 HNC patients treated at Washington University in St. Louis using IMRT for the upper neck only to spare the parotid glands with a matched anterior low neck field (Chao et al. 2003). Twenty-eight percent of recurrences in this series (five patients) occurred in the supraclavicular area, underscoring the importance of avoiding underdosing of low neck nodal levels in patients in whom these regions are at high risk, such as those with clinical nodal involvement in levels III or IV or with bulky nodal involvement of level II. In cases where the lower neck is at low risk, a split-neck technique with a larynx block does confer certain potential benefits over a comprehensive IMRT plan, such as reduced dose to the glottic larynx, reduced labor-intensiveness of target delineation, short treatment time, less monitor unit delivery, and less daily setup deviation in the low neck (Eisbruch and Gregoire 2009). These benefits, however, may come at the cost of reduced dose certainty to targets in the low neck targets and particularly at the IMRT field and low neck field junction, potentially resulting in over- or underdosing in these regions. Additionally, we have found that in cases where the low neck is at low risk, a comprehensive IMRT plan which prioritizes avoidance of the glottic larynx, inferior pharyngeal constrictor, and upper esophagus can consistently reduce the mean dose to these structures to 20–30 Gy over 35 fractions, which is comparable to the dose received when using a split-neck technique. In cases where the low neck is a high risk, a comprehensive IMRT plan may result in higher doses to these avoidance structures, but with the benefit of more accurate coverage of at-risk targets in the low neck, reduced risk of underdosing at the split-neck field junction, and the ability to partially spare OARs such as the larynx, cricopharyngeus, esophagus, and brachial plexus while delivering high doses to gross disease in the low neck (i.e., level IV). Therefore, at our institution, we employ the use of a single comprehensive IMRT plan rather than a matched anterior low neck field for treatment of the low neck and strongly recommend this approach in cases where the low neck targets are grossly involved or at high risk of subclinical metastases.
4.3.4.5 Preservation of the Swallowing Structures
Dysfunction of the swallowing structures is a common cause of severe acute and late toxicity after head and neck radiotherapy and is perhaps the strongest determinant of long-term quality of life in HNC survivors (Hunter et al. 2013; Langendijk et al. 2008; Machtay et al. 2008; Terrell et al. 2004). Radiotherapy dose to the swallowing structures, specifically the pharyngeal constrictor (PC) muscles, has been implicated as a primary treatment-related predictor of swallowing dysfunction after chemoradiotherapy (Eisbruch et al. 2011; Feng et al. 2007; Popovtzer et al. 2009). Therefore, avoidance of dose to the PCs should be attempted where possible, although this goal must be balanced against the need for adequate coverage of the RP nodes, due to their frequent involvement in cancers of the nasopharynx, oropharynx, and hypopharynx (Chung et al. 2011; Coskun et al. 2011; Eisbruch et al. 2004a; Hasegawa and Matsuura 1994; King et al. 2000). The RP nodes consist of lateral and medial groups, with the medial RPs located near midline close to the center of the PCs. As metastatic involvement of the medial RP nodes is rare in HNC (with the exception of tumors that invade the posterior pharyngeal wall), it has been postulated that these nodes may be excluded from the CTV such that high dose to adjacent PCs can be avoided. This hypothesis was tested at the University of Michigan in a prospective study of 73 patients with locally advanced (stages III–IV) oropharynx cancer designed to assess the clinical and functional results of chemoradiotherapy using IMRT to spare the important swallowing structures (Feng et al. 2010). IMRT planning was performed with the intent of sparing non-involved parts of the swallowing structures, namely, the pharyngeal constrictors, glottic larynx, supraglottic larynx, and esophagus as well as the oral cavity and major salivary glands (Fig. 4.5). At a median follow-up of 36 months, 3-year disease-free and locoregional recurrence-free survival was 88 and 96 %, respectively. At 1 year after completion of therapy, observer-rated dysphagia was absent or minimal (scores 0–1) in all except four patients: one who was feeding-tube dependent and three who required soft diet. These outcomes clearly indicate that IMRT aimed at reducing dose to the swallowing structures in oropharynx cancer can safely minimize dysphagia while maintaining high rates of locoregional tumor control.
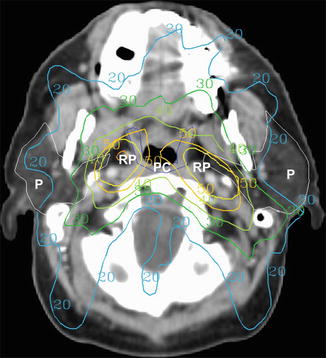

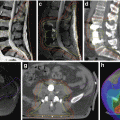
Fig. 4.5
Example of an intensity-modulated radiotherapy plan for a patient with a left base of the tongue T1 N2a squamous cell carcinoma. Dose distributions (displayed in Gy) are labeled. Note the avoidance of high dose to the medial pharyngeal constrictor muscles (PC, blue contour) while treating the lateral retropharyngeal (RP) node planning target volumes (yellow contour). The bilateral parotid glands (P, white contour) are also largely spared
4.3.4.6 Summary
In summary, these analyses suggest the additional recommendations shown in Table 4.3 for target volume delineation in IMRT and augment those already understood.
Table 4.3
Additional recommendations for target delineation based on recent studies
The lateral RP nodes should be treated bilaterally in all cases of nasopharyngeal cancer, cancers involving the posterior pharyngeal wall, and most cases of locally advanced oropharyngeal cancer |
Treatment limited to the ipsilateral RP nodes in oropharyngeal cancer is at present recommended only in cases of small, well-lateralized oropharyngeal tumors with N0 or small-volume N1 disease. In the future, however, this recommendation may be extended to also include those cancers without contralateral nodal involvement (i.e., N0–N2b), pending validation of recently reported evidence suggesting that this practice may not increase the risk of marginal or out-of-field locoregional failures (Spencer et al. 2012) |
The superior extent of the CTV for the lateral RP nodes should extend cranially beyond C1 to the skull base |
The medial RP nodes are not included in the CTV unless the posterior pharyngeal wall is involved; thus, the medial parts of the pharyngeal constrictors can be spared from effects of high radiotherapy doses |
In patients who have had major neck surgery more than 1 year prior to treatment, IMRT should be used with extreme caution due to the uncertainty regarding their targets |
When the low neck is at high risk, such as in patients with significant upper neck nodal involvement, particular attention must be paid to the low neck targets to ensure appropriate dose delivery. This is of paramount importance when using a low anterior photon field matched to an upper neck IMRT plan |
4.4 The Postoperative Case: Defining the Clinical Target Volumes
In surgically treated head and neck squamous cell carcinoma with pathological risk factors in either the primary or regional sites of involvement to warrant adjuvant radiotherapy (either with or without concurrent chemotherapy), the surgical specimen provides valuable information that should be helpful in determining the neck levels at risk. Resection of the primary site, neck dissection, and surgical reconstruction disrupts some of the anatomical landmarks used to define the borders between the levels. The surgical bed, however, remains apparent on CT and should be encompassed entirely within the CTV. As it is often impossible to distinguish between the primary tumor resection site and the adjacent neck dissection bed, they are encompassed within a unified CTV. Sites of positive margins or extracapsular extension are delineated generously as a higher-risk CTV which should receive a higher radiotherapy dose (as described below). Additionally, irrespective of whether they were included in the neck dissection, nodal levels deemed at risk based upon the primary site location or extent of neck involvement (i.e., level VI in cases of pathological level IV involvement) should be included in the CTV (see Sect. 4.3.2). For malignant salivary gland tumors which are primarily managed surgically, those with tumors that are high grade, T3 or T4, recurrent, resected incompletely or with close margins, have perineural or gross nerve invasion, or have lymph node metastases should receive adjuvant RT (Chen et al. 2007a, c; Terhaard et al. 2005). CTV delineation guidelines for the primary site and neck are as detailed above (see Sects. 4.2.2, 4.3.2, and 4.3.3, respectively).
Recent surgical series of transoral robotic surgery (TORS) and transoral laser microsurgery (TLM) for oropharynx cancer with favorable outcomes after surgery alone, even in stage III and IV disease, have proposed that postoperative radiotherapy can be safely omitted in cases with traditional indications for adjuvant RT after TORS or TLM (Grant et al. 2009; Weinstein et al. 2012). These studies, however, are limited by their small patient numbers, patient selection biases, lack of matched comparator groups of patients who received RT, and in the case of the TLM studies, their retrospective nature. Until omission of RT in such patients is supported by higher-level evidence, the use of postoperative RT to both the primary site and neck for those with appropriate indications should remain the standard of care.
4.5 Target Doses and Treatment Techniques
4.5.1 Doses
At our institution, our current treatment policy is to deliver 70 Gy to the GTV in 35 fractions (2.0 Gy per fraction). The high-risk CTV, which is around the GTV and the first echelon nodes, receives 59–63 Gy (at 1.7–1.8 Gy per fraction, biologically equivalent to 54–60 Gy at 2.0 Gy per fraction). The lower-risk areas receive 56–59 Gy (1.6–1.7 Gy per fraction, biologically equivalent to approximately 49–54 Gy at 2.0 Gy per fraction). These specifications are very similar to the policies at UCSF, Washington University in St. Louis, University of Iowa, and MSKCC (Chao et al. 2003; Lee et al. 2003; Setton et al. 2012; Yao et al. 2005). Concurrent chemotherapy for patients with locally advanced (stages III–IV) disease is strongly recommended for fit patients based upon level I evidence (Denis et al. 2004; Forastiere et al. 2003; Pignon et al. 2009). For patients receiving concurrent RT, we favor the use of the standard fractionation scheme above rather than accelerated radiotherapy, which has failed to show a benefit in terms of locoregional control or survival in phase III studies (Ang et al. 2010; Bourhis et al. 2012). In patients with locally advanced disease who do not receive concurrent chemotherapy, due typically to comorbid illness or other contraindication, accelerated or hyperfractionated radiotherapy is recommended, on the basis of multiple randomized trials demonstrating improvement in locoregional control and a meta-analysis demonstrating an improvement in overall survival compared with conventional radiotherapy (Bourhis et al. 2006; Fu et al. 2000; Horiot et al. 1992; Overgaard et al. 2003). Our institutional preference in this situation has been to use the DAHANCA regimen of 6 fractions per week (with the sixth fraction given either on Saturday or as a second fraction 1 day per week given at least 6 h apart) to deliver our standard fractionation scheme above while accelerating the course of therapy by 1 week (Overgaard et al. 2003).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree