The clinical evaluation and management of HCC require a comprehensive, multidisciplinary approach that involves cancer surveillance and consideration of both surgical and medical therapies.
The implementation of such an approach has resulted in increased survival rates for HCC. The therapeutic approach for HCC can vary widely depending on the extent of disease: from potentially curative surgical resection and/or ablation for small localized tumors to liver transplantation or newer biologic therapies for more advanced disease. Advances in minimal invasive therapies, such as radiofrequency (RFA), microwave (MW) ablation, and transarterial chemoembolization (TACE), also continue to play a vital role in the management of more advanced stages and in pre- and perioperative transplant patients.
4.3 Staging Systems
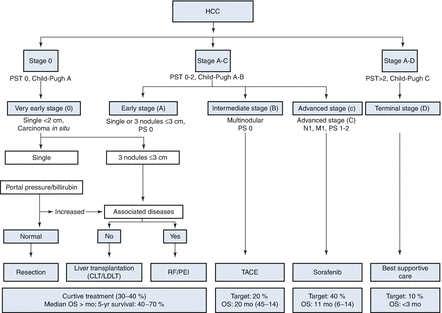
Disease staging is particularly important in the management of HCC because it helps to predict prognosis and determine appropriate treatment options; the most effective staging systems incorporate information about both cancer stage and liver function, which is often affected by the underlining liver disease. The Child–Turcotte–Pugh (CTP = TAB IIa/IIb) model is primarily an assessment of liver function and is intended to predict prognosis and stratify disease severity to facilitate transplant allocation [9]. While still used as a complementary tool to help with treatment decisions or evaluate progression and/or regression of disease, the CTP model has largely been replaced by the Model for End-Stage Liver Disease (MELD) score [10, 11]. MELD was originally developed at the Mayo Clinic and at that point was called the “Mayo End-Stage Liver Disease” score [12]. It was derived in a series of patients undergoing TIPS procedures. The score turned out to be predictive of prognosis in chronic liver disease in general, and – with some modifications – came to be applied as an objective tool in assigning need for a liver transplant. Higher MELD scores reflect more severe disease, poorer prognosis, and greater likelihood of liver transplantation, barring any absolute contraindications to transplantation [13–16]. While patients with HCC may be granted exception points that are added to their scores, the MELD system was not designed to assess HCC disease severity, and it does not provide good prognostic classification for these patients. The four major HCC staging systems include the American Joint Committee on Cancer’s tumor-node-metastasis (TNM) model, the Okuda classification model, the Cancer of the Liver Italian Program (CLIP) score, and the Barcelona Clinic Liver Cancer (BCLC) staging system. The BCLC staging system has emerged as the most accurate and comprehensive cancer model to show consistent prognostic determination. The Barcelona Clínic Liver Cancer classification divides HCC patients in five stages (0, A, B, C, and D) according to preestablished prognostic variables and allocates therapies according to treatment-related status (Table 4.2) [17–19]. Thus, it provides information on both prognostic prediction and treatment allocation. Prognosis prediction is defined by variables related to tumor status (size, number, vascular invasion, N1, M1), liver function (Child–Pugh’s), and health status (ECOG) (Tables 4.3a and 4.3b). Treatment allocation incorporates treatment-dependent variables, which have been shown to influence therapeutic outcome, such as bilirubin, portal hypertension, or presence of symptoms – ECOG. While future studies incorporating genomic and proteomic profiles of patients and their cancers will provide even more accurate prognostic data and more individualized therapy, the BCLC model is currently the most comprehensive and widely accepted staging system for HCC.
Table 4.3a
Child–Pugh score system
Measure | 1 point | 2 points | 3 points |
|---|---|---|---|
Total bilirubin,μmol/l (mg/dl) | <34 (<2) | 34–50 (2–3) | >50 (>3) |
Serum albumin, g/l | >35 | 28–35 | <28 |
PT INR | <1.7 | 1.71–2.30 | >2.30 |
Ascites | None | Mild | Moderate to severe |
Hepatic encephalopathy | None | Grade I–II (or suppressedwith medication) | Grade III–IV(or refractory) |
Table 4.3b
Child–Pugh score classification
Points | Class | One-year survival (%) | Two-year survival |
|---|---|---|---|
5–6 | A | 100 | 85 |
7–9 | B | 81 | 57 |
10–15 | C | 45 | 35 |
4.4 Prognosis
The prognosis of advanced hepatocellular carcinoma (HCC) remains poor, particularly for patients with portal vein tumor thrombosis and extrahepatic metastases (median survival: 3–6 months)
The Tokyo index is a new and simple indicator for prognosis for survival
Tokyo score | |||
|---|---|---|---|
Parameter | 0 | 1 | 3 |
Albumin (g/dl) | >3.5 | 2.8–3.5 | <2.8 |
Bilirubin (mg/dl) | <1 | 1–2 | >2 |
Tumor size (cm) | <2 | 2–5 | >5 |
Tumor foci | <3 | 1–3 | >3 |
Patients with a score up to 2 do have a relative good prognosis. Patients with a total score between 4 and 6 do have a 2-years survival expectation of 50 %.
4.5 Therapy
In oncology, the benefits of treatments should be assessed through randomized controlled trials and meta-analysis. Few medical interventions have been thoroughly tested in HCC, in contrast with other cancers with a high prevalence worldwide, such as lung, breast, colorectal, and stomach cancer. As a result, the strength of evidence for most interventions in HCC is far behind the most prevalent cancers worldwide. The level of evidence for efficacy according to trial design and endpoints for all available treatments in HCC and the strength of recommendations according to grade are summarized in Table 4.4.
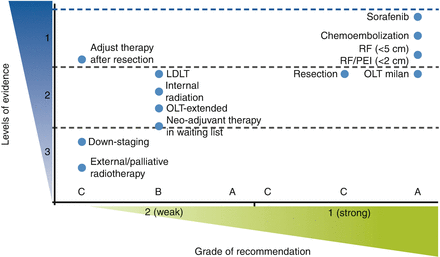
Table 4.4
Representation of EASL–EORTC recommendations for treatment according to levels of evidence (NCI classification) and strength of recommendation (grade system) [22]

Recommendations in terms of selection for different treatment strategies should be based on evidence-based data in circumstances where all potential efficacious interventions are available. However, multidisciplinary HCC tumor boards, including hepatologists, surgeons, oncologists, radiologists, interventional radiologists, pathologists, and translational researchers, should discuss any single HCC patient according to the international guidelines, and treatment strategies should be adapted to local regulations and/or team capacities and cost–benefit strategies.
4.5.1 Surgical Approach
The best treatment options with curative intent for patients with HCC are liver resection or transplantation, although the role of hepatic ablative therapies has also been recognized. Surgical resection has emerged as the primary treatment in carefully selected patients of HCC. With the advances in surgical and radiological techniques, the perioperative mortality has been reduced to less than 5 % depending on the extent of resection and hepatic reserve. Modern standards of HCC resection in cirrhotic patients are defined as follows: expected 5-year survival rates of 60 %, with a perioperative mortality of 2–3 % and blood transfusion requirements of less than 10 % [23–27]. Anatomic resections aiming at 2 cm margins provide better survival outcome than narrow resection margins <1 cm [28] and are recommended only in case that the maintenance of appropriate function to the remnant liver volume is ensured. In patients properly selected according to liver functional status, the main predictors of survival are tumor size, tumor number, presence of microsatellites, and vascular invasion [29]. The Japanese Nationwide Survey has shown that a cutoff below 2 cm is an independent predictor of survival in a series of thousands of patients [30]. Five-year survival rate for patients with HCC ≤ 2 cm was of 66 %, compared with 52 % for tumors 2–5 cm and 37 % for tumors >5 cm. Multinodularity also predicts survival, with 5-year survival rates after resection of single tumors of 57 and 26 % for three or more nodules, respectively.
Liver transplantation is the first treatment choice for patients with small multinodular tumors (≤3 nodules ≤ 3 cm) or those with single tumors ≤5 cm and advanced liver dysfunction. Theoretically, transplantation may simultaneously cure the tumor and the underlying cirrhosis. The role of liver transplantation as the mainstay of treatment for the majority of patients with HCC has evolved in the last few decades. Historically, the Milan criteria have been considered the gold standard for selecting patients: single HCC ≤5 cm or up to three nodules ≤3 cm [31]. Following these criteria and according to modern standards, perioperative mortality, 1– and 5–year mortality are expected to be 3 %, ≤10 %, and ≤30 %, respectively. Living-donor liver transplantation has emerged as a way to expand the donor pool and has influenced the role of transplantation for HCC, especially in communities with little access to cadaveric transplantation. Salvage transplantation is an alternative option as it allows a window for the biologically less favorable lesions to declare tumor behavior. Salvage transplantation also decreases the burden on transplant resources. Three years survival expectation was 60–80 %.
4.5.2 Systemic Therapy
Systemic chemotherapy does not play a central role in the treatment of HCC due to the issue of low sensitivity for chemotherapeutic agents and the difficulties in administering a sufficient dose due to chronic liver dysfunction. Systemic treatment by mean of biologicals is the new frontier for advanced stage HCC. Sorafenib, an oral protein kinase inhibitor, is a systemic drug that has been licensed for the treatment of hepatocellular carcinoma (HCC). An international, phase III, placebo-controlled trial could show a minimal but demonstrated advantage in the median OS for the sorafenib group.
4.5.3 Minimally Invasive Locoregional Therapies
Locoregional hepatic tumor therapies include intra-arterial, percutaneous, and external therapies:
Intra-arterial Therapies
1.
Hepatic arterial infusion (HAI)
2.
Transarterial chemoembolization (TACE)
3.
Transarterial embolization (TAE)
4.
Y90 Radio embolization (Y90RE)
5.
Percutaneous hepatic chemoperfusion (PHP)
Percutaneous Therapies
1.
Percutaneous ethanol injection (PEI)
2.
Local ablative techniques (radiofrequency ablation, RFA/microwave ablation, MWA/laser-induced thermotherapy, LITT)
3.
Combined therapies (usually intra-arterial and local ablative
External Therapies
1.
External radiation therapy (EBRT)
2.
High-intensity focused ultrasound
4.5.3.1 Intra-arterial Therapies
Clinical conditions:
Patients with big uninodular or multinodular HCC
Sufficient liver function
No infiltration of other big vessels
No distal metastases influencing the prognosis
Hepatic Arterial Infusion (HAI)
Chemotherapeutic Agents: 5-Fluorouracile, Cisplatin, Mitomycin C
The concept of regional chemotherapy for hepatic metastases via HAI is based on several principles. First, hepatic tumors (both primary and metastatic ones) derive their blood supply from the hepatic artery, while normal hepatocytes are perfused mostly from the portal circulation [32]. Thus, infusion of chemotherapy via the hepatic artery could achieve toxic levels in tumor cells with relative sparing of normal hepatic parenchyma. Second, extraction of drug from the hepatic arterial circulation via the first-pass effect can result in high local concentrations and minimal systemic toxicity. The ideal agent should have a high dose–response curve, high extraction rate, and rapid total body clearance once infusion is discontinued. Intra-arterial chemotherapy is one of the possible treatment options for patients with advanced HCC not candidate for hepatic resection, percutaneous ablation, and transcatheter arterial chemoembolization. Patients with advanced HCC are increasingly treated in Japan with hepatic arterial infusion chemotherapy (HAIC). HAIC may provide moderate therapeutic efficacy and survival benefit with substantially tolerable toxicity profiles in patients with advanced HCC.
A dedicated arterial infusion catheter is placed through the left subclavian artery with the tip located into the coiled GDA. A side hole is made at the level of proper hepatic artery in order to deliver the drug into the arterial bloodstream. Proximal end of infusion catheter is connected with a reservoir (port) which is surgically placed in a subcutaneous pocket, below the clavicle. In BCLC treatment strategy flowchart, selective intra-arterial chemotherapy is not recommended for the management of HCC (evidence 2A; recommendation 2B).
Transarterial Chemoembolization (TACE)
Chemotherapeutic Agents: Doxorubicin, Cisplatin, Mitomycin C
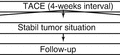
Chemoembolization is the most widely used primary treatment for unresectable HCC [30, 33, 34] and the recommended first-line therapy for patients at intermediate stage of the disease [20, 35, 36]. HCC has an intense neo-angiogenic activity during its progression. The rationale for TACE is that the intra-arterial infusion of a cytotoxic agent followed by embolization of the tumor-feeding blood vessels will result in a strong cytotoxic and ischemic effect.
TACE should be distinguished from the Lipiodol TACE (cTACE), drug-eluting beads TACE (debTACE), and bland embolization (TAE, and micro-bland TAE).
cTACE combines transcatheter delivery of chemotherapy emulsioned with Lipiodol followed by embolization of the feeding arteries. Chemoembolization achieves partial responses in 15–55 % of patients and significantly delays tumor progression and macrovascular invasion. Survival benefits were obtained in two studies [37, 38]
Meta-analysis of some RCT showed a beneficial survival effect of TAE/cTACE in comparison to the control group [36]. Sensitivity analysis showed a significant benefit of cTACE with cisplatin or doxorubicin in four studies, but none with embolization (using old embolic materials) alone in three studies. Overall, the median survival for intermediate HCC cases is expected to be around 16 months, whereas after chemoembolization the median survival is about 20 months. As a result of these investigations, TACE has been established as the standard of care for patients who meet the criteria for the intermediate stage of the BCLC staging system.
Treatment-related deaths are expected in less than 2 % of cases, and the best candidates are patients with preserved liver function and asymptomatic multinodular tumors without vascular invasion or extrahepatic spread. Patients should present relatively well-preserved liver function (mostly Child–Pugh A or B7 without ascites). Patients with liver decompensation or more advanced liver failure should be excluded since the ischemic insult can lead to severe adverse events [39]. There is no good evidence for which is the best chemotherapeutic agent and the optimal re-treatment strategy. Superselective chemoembolization is recommended to minimize the ischemic insult to non-tumoral tissue.
cTACE, debTACE, and TAE are usually performed through the femoral artery percutaneous approach. A selective angiography of proper hepatic artery has to be performed in order to define the liver vasculature and detect the tumor-feeding vessels. With the help of selective catheters and microcatheters, a superselective embolization of tumor-feeding arteries may be achieved, sparing the unaffected areas of the liver parenchyma. Endpoint for a better result should be the vascular shutdown to the tumor. Despite selecting the patients and performing a superselective embolization, TACE is not without risks. Complication may range from postembolization syndrome (of variable intensity) to liver abscesses, hepatic insufficiency, and ischemic cholecystitis, or cases of death have even been also described.
DEBTACE. The ideal TACE scheme should allow maximum and sustained intratumoral concentration of the chemotherapeutic agent with minimal systemic exposure, along with calibrated tumor vessel obstruction. DEBTACE is performed by injecting microspheres loaded with antiblastic drug, such as doxorubicin. Unlikely to the cTACE, where the injected drug is quickly release into the systemic circulation, drug-eluting beads provide a gradual release of the chemotherapy agent into the tumor, reducing the systemic side effect and maximizing the local efficacy against tumor cells. Embolic microspheres have the ability to sequester chemotherapeutic agents and release them in a controlled mode over a 1-week period. This strategy has been shown to increase the local concentration of the drug with negligible systemic toxicity [40]. However, a randomized phase II study comparing TACE and TACE-DEB reported a nonsignificant trend of better antitumoral effect [41] in the latter arm.
Transarterial Embolization (TAE)
In the majority of published studies on HCC treatment with TAE, the reported embolic agent is gelatine sponge, which may induce only temporarily ischemia and without distal tumor vessel embolization. Only recently, few new studies on new embolic agents, such as resin or gelatine microspheres, are available. Even if there is no evidence for a better survival benefit from DEB TACE than TACE and also TAE, if performed with small particles (40/100 μm), there is an increasing general consensus about the need to use the smallest available particles in treating HCC, in order to achieve a better, durable, and deeper embolic effect, independently by the use of drug or not [42–45]. Few papers on HCC treatment with TAE, using very small particles, reported an interesting safety profile with local results comparable with DEBTACE/TACE series [46]. However, based on data coming from old papers on TAE with gelatin sponge, BCLC doesn’t recommend the use of TAE for HCC.
Y90 Radio Embolization (Y90RE)
Radioembolization is defined as the infusion of very small (<40 μm) microspheres containing yttrium-90 (90Y) [47–49] into the hepatic artery. Due to the hypervascularity of HCC, intra-arterial injection of microspheres will be preferentially delivered to the tumor-bearing area and selectively emit high-energy, with a low-penetrating radiation to the tumor. This treatment should be reserved only to centers with sophisticated equipments and trained interventional radiologists in cooperation with nuclear medicine specialists, in order to reduce the potential risk of possible serious side effects: severe lung shunting and intestinal radiation should be prevented prior to the procedure. This treatment can be safely used in patients with portal vein thrombosis, where it seems to obtain the best clinical results [48]. Recently, some studies reported a median survival time of 17.2 months for patients at intermediate stages and 12 months for patients at advanced stages and portal vein invasion [48–50]. Objective response rates ranged from 35 to 50 % [47–49]. Around 20 % of patients present liver-related toxicity and 3 % treatment-related death [47]. Despite the amount of data reported, there are no RCT testing the efficacy of 90Y radioembolization compared with chemoembolization or sorafenib in patients at intermediate or advanced stage, respectively. Further research trials are needed to establish a competitive efficacy role in this population (BCLC = evidence 2A; recommendation 2B)
Percutaneous Hepatic Chemoperfusion (PHP)
Percutaneous hepatic perfusion (PHP) is a regionalized, minimally invasive approach to cancer treatment currently undergoing phase II and phase III clinical testing in melanoma, CRC, and NET metastatic patients. PHP may treat a variety of hepatic tumors, including HCC, by isolating the liver and exposing the organ to high-dose chemotherapy [51]. As demonstrated in clinical trials, patients treated by PHP can tolerate much higher doses of chemotherapeutic agents than those receiving traditional systemic chemotherapy without increased toxicities.
Using a system of catheters and filters, PHP isolates the liver from the circulatory system and infuses a chemotherapeutic agent directly to the liver via the hepatic artery. The venous effluent from the liver is then filtered outside of the body and the filtered blood is returned into the jugular vein. PHP is a repeatable procedure and can be performed in an operating room or a radiology suite under general anesthesia. There are very few experiences in the treatment of HCC patients; however, the complexity of this revolutionary technique represents the main limitation. Further studies and a longer experience are needed before to treat HCC patient with PHP outside protocol studies.
4.5.4 Study Results: Neoadjuvant Therapies (HAI/Chemoembolization)
Author | N | Concept | Intra-arterialtherapy | RR (%) | Mediansurvival(mo) | Yearssurvival (%) |
|---|---|---|---|---|---|---|
Gerundaet al. (2000) [52] | 89 | TACE + LR vs. LR vs. TACE | 1×: 50 mg epirubicin+ Gelfoam | ND | Overall survival: TACE + LR vs. TACE/LR: p < 0.05 | 1 y: 85 vs. 71 vs. 68 5 y: 43 vs. 38 vs. 0 |
Graziadeiet al. (2003) [53] | 48 | TACE + LT | 70 mg epirubicin + Lipiodol (±PVA particles) every 6–8 weeks | CR: 30 PR: 67 | ND | 1 y: 98 2 y: 98 5 y: 94 |
Yao et al. (2005) [54] | 30 | TACE ± RFA ± PEI + LT | ND | Down staging:70 | ND | 1 y: 89 2 y: 82 |
Bharat et al. (2006) [55] | 100 | TACE (78 %), RFA (11 %), PEI (2 %), TACE + RFA (9 %) + LT vs. LT | 50 mg cisDDP + 20 mg doxorubicin + 10 mg MMC+ particles every 4–6 weeks | path RR: signif. advantage for neoadj. therapy | 5 y OS(%): 82 vs. 52 (no difference in pT0 and pT1) | ND |
Obed et al. (2007) [56] | 74 | TACE + LT vs. TACE vs. no therapy | 50 mg epirubicin + Lipiodol every 6 weeks | After TACE: 29 PD: 70 | 92 vs. 8 vs. 4 | ND |
Zangos et al. (2007) [57] | 48 | TACE + LITT | 10 mg/m2 MMC + Lipiodol + DSM 3× every 4 weeks | RR: 67 SD: 25 PD: 8 | 36 | ND |
Hoffmann et al. (2008) [58] | 208 | TACE ± sorafenib + LT | 4× carbo-DDP + Lipiodol | |||
Zhou et al. (2009) [59] | 108 | TACE vs. control | 3× 1,000 mg 5-FU + 20 mg MMC + 5 mg cisDDP + Lipiodol every 4–9 weeks | Path. RR: ≤50 %: 40.4 vs. 94.6 50–100 %: 59.6 vs. 5.4 (p < 0.01) | ND | DfS(1 y, 3 y, 5 y): 49, 26, 13 vs. 39, 21, 9 OS (1 y, 3 y, 5 y): 73, 40, 31 vs. 70, 32, 21 p > 0.05 |
Choi et al. (2009) [60] | 16 | TACE + radiation + LR | 50 mg doxorubicin + Lipiodol + Gelfoam median: 3×/patient | 12 CR: 0 PR: 2 PD: 3 | 13 | ND |
Schaudtet al. (2009) [61] | 27 | TACE/TACE + PEI/LITT + LT | 10 mg MMC + Lipiodol + DSM every 3–6 weeks | TACE (N = 15): PR/SD: N = 14 | OS (TACE vs. non-TACE): 82 vs. 61 % | ND |
Recommendation (for borderline operable tumors):
Concept | Intra-arterial chemoembolization |
Access | Catheter via A. femoralis in A. hepatica propria |
Therapy | 50 mg/m2 doxorubicin + 300 mg amilomer (over 20–30 min) |
± 60 mg/m2 cisplatin (over 20–30 min) | |
2× every 3–4 weeks | |
LR/LT after further 4 weeks |
Further clinical studies are required.
4.5.5 Study Results: Adjuvant Therapy (HAI/Chemoembolization)
Author | N | Concept | Intra-arterial therapy | Median survival (mo) | Years survival/DfS (%) |
|---|---|---|---|---|---|
Lai et al. (1998) [62] | 66 | LR + TACE + IV chemotherapy vs. LR (control) | 3× 10 mg cisDDP + Lipiodol + 40 mg/m2 doxorubicin IV every 2 months | ND | DfS (1, 2, 3 y): 50, 36, 18 vs. 69, 53, 48 (p = 0.04) |
Ono et al. (2001) [63] | 108 | HAI/IV vs. control (meta-analysis of 3 protocols) | 1. 1× 40 mg/m2 epirubicin + oral 300 mg/d tegafur vs. control 2. 1× 40 mg/m2 epirubicin + IV 40 mg/m2 epirubicin every 3 months + 300 mg/d carmofur (2 years) vs. control 3. IV 40 mg/m2 epirubicin every 2 months (1 year) vs. control | OS: significant advantage in patients without adjuvant treatment p = 0.02 | DfS (3, 5 y): 37, 28 vs. 42, 26 p = 0.324 |
Wen et al. (2006) [64] | 28 | LR + HAI | d1: 250 mg FUDR d4: 10 mg doxorubicin d7: 4 mg MMC 8 cycles (1st and 2nd year after resection) | ND | 1 y: 11 3 y: 7 5 y: 5 |
Li et al. (2006) [65]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|