Hemoglobin E thalassemia accounts for about one-half of all cases of severe beta thalassemia. There is marked variability in its clinical severity ranging from an asymptomatic to a transfusion-dependent phenotype. The phenotypic variability and inadequate longitudinal data present challenges in determining the optimal management of patients. This article summarizes findings on the natural history of Hemoglobin E thalassemia and some factors responsible for its clinical heterogeneity. Major genetic factors include the type of beta thalassemia mutation, the co-inheritance of alpha thalassemia, and polymorphisms associated with increased synthesis of fetal hemoglobin. Other factors, including response to anemia, and the influence of infection with malaria and other environmental influences, may be important. The remarkable variation and instability of clinical phenotypes in Hemoglobin E thalassemia require individual management plans for each patient, which should be reassessed over time.
Epidemiology
Worldwide, hemoglobin E (HbE) thalassemia accounts for about one-half of all cases of severe β-thalassemia, with the highest frequencies observed in India, Bangladesh, and throughout southeast Asia in regions of Thailand, Laos, and Cambodia.
Hemoglobin E β-thalassemia (HbE/β-thalassemia) is causing an increasingly severe public health problem throughout the Indian subcontinent and parts of southeast Asia. Its carrier frequencies are up to 60% in parts of northeast Thailand and Cambodia, it is common for individuals in this region to inherit both HbE and β-thalassemia. In Thailand, 3000 children are born annually with HbE/β-thalassemia and there are more than 100,000 patients in the population. In southern China, gene frequencies are approximately 4% for β-thalassemia and for HbE, resulting in thousands of affected patients. HbE thalassemia accounts for more than half of the cases of severe β-thalassemia in Indonesia, and for about one-third of those in Sri Lanka.
Although, in the past, HbE/β-thalassemia was rarely diagnosed in North America and Europe, it has become the most common form of β-thalassemia identified on state newborn screening programs.
The prevalence of HbE in California parallels the increase in Asian births in that state: the carrier state is observed in 1 in 4 births Cambodian origin and in 1 in 9 babies of Thai/Laotian origin.
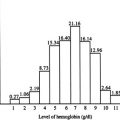
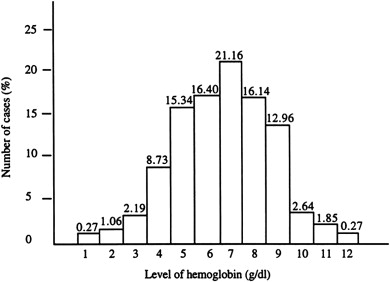
There is a widely disparate range of clinical and hematological parameters in affected patients ( Fig. 1 ). Although studies are limited, the natural history of HbE/β-thalassemia may change over time in individuals. The phenotypic variability, the way the later course of the illness cannot be predicted in the first few years of life, and the lack of knowledge of the natural history of the disease combine to make the management of HbE/β-thalassemia particularly challenging.
Pathophysiology
HbE thalassemia results from coinheritance of a β-thalassemia allele from one parent, and the structural variant, HbE, from the other. HbE results from a G→A substitution in codon #26 of the β-globin gene, which, as well as producing a structurally abnormal hemoglobin, activates a cryptic splice site that causes abnormal messenger RNA (mRNA) processing. Because the usual donor site has to compete with this new site, the level of normally spliced mRNA, β E , is reduced and the abnormally spliced mRNA is nonfunctional because a new stop codon is generated. As a result, HbE is synthesized at a reduced rate, and behaves like a mild form of β-thalassemia.
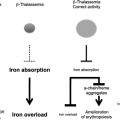
The pathophysiology of HbE/β-thalassemia is related to many factors including reduced β chain production resulting in globin chain imbalance, ineffective erythropoiesis, apoptosis, oxidative damage, and shortened red cell survival. The instability of HbE is a minor factor in the overall pathophysiology of this disorder except during febrile events in which this instability results in accelerated hemolysis.
Pathophysiology
HbE thalassemia results from coinheritance of a β-thalassemia allele from one parent, and the structural variant, HbE, from the other. HbE results from a G→A substitution in codon #26 of the β-globin gene, which, as well as producing a structurally abnormal hemoglobin, activates a cryptic splice site that causes abnormal messenger RNA (mRNA) processing. Because the usual donor site has to compete with this new site, the level of normally spliced mRNA, β E , is reduced and the abnormally spliced mRNA is nonfunctional because a new stop codon is generated. As a result, HbE is synthesized at a reduced rate, and behaves like a mild form of β-thalassemia.
The pathophysiology of HbE/β-thalassemia is related to many factors including reduced β chain production resulting in globin chain imbalance, ineffective erythropoiesis, apoptosis, oxidative damage, and shortened red cell survival. The instability of HbE is a minor factor in the overall pathophysiology of this disorder except during febrile events in which this instability results in accelerated hemolysis.
Understanding the phenotypic heterogeneity of HbE/β-thalassemia
The extraordinary clinical heterogeneity of HbE/β-thalassemia is poorly understood. The condition may present as a mild, asymptomatic anemia or a life-threatening disorder that may lead to death from anemia in the early first years of life. In an analysis conducted in Sri Lanka, patients with HbE/β-thalassemia would have been expected to account for 40% of the patients affected by thalassemia at any given time but, based on the Hardy-Weinberg distribution, many fewer cases of HbE/β-thalassemia were observed, likely reflecting the mildness of some cases of HbE and its variable clinical course.
The phenotype of HbE/β-thalassemia seems to be unstable. Presently, little is known about the clinical course of older patients. Limited data indicate that patients develop worsening anemia with age.
Longitudinal studies of affected children in Sri Lanka have emphasized the remarkable instability of their phenotypes, particularly in the first 10 years of life, during which there was a variable and changing pattern of anemia, erythroid expansion, and associated growth failure. By contrast, the phenotype became more stable later in development, and it was frequently possible to stop blood transfusion in a proportion of older patients, with no deleterious effects on their further development and function. One of the most important findings identified in the modified natural history study of HbE/β-thalassemia reported from Sri Lanka was that, although about a quarter of patients were not receiving regular blood transfusions, and the rest were maintained on regular or on-demand transfusions, the pretransfusion steady-state hemoglobin concentrations (on average 7.0 g/dL) were not clinically very different from those patients who had never begun a regimen of transfusions (mean 6.1 g/dL). A considerable phenotypic heterogeneity of these patients occurred within a narrow range of hemoglobin values, with the mean difference between the mildest and most severe groups approximately 1 to 2 g/dL.
The lack of a standardized, robust classification of disease severity is a major impairment in understanding the clinical spectrum of the disease and whether it changes with age.
Clinical severity categories of HbE/β-thalassemia
Investigators have attempted to categorize disease severity with the assignment of patients to severe and mild groups, between which putative genetic and environmental factors can be compared. Premawardhena and colleagues classified 109 patients with HbE/β-thalassemia aged 1 to 51 years into very mild to very severe groups. About one-quarter of patients were transfusion independent, whereas the remainder had been maintained on regular or intermittent (on demand) transfusion. In an analysis of the patient data, there was little objective difference in the baseline data between the transfused and nontransfused cohorts. In most of the cases, it was unclear whether transfusions were necessary. Therefore, it was decided to stop transfusion in as many patients as possible, and observe the patients closely. This cohort has been prospectively followed for more than 8 years.
These patients were classified into 5 groups of severity ranging from mild to severe ( Table 1 ). Group 1 included those patients who had undergone only minimal transfusion and had normal growth and sexual maturity; group 1 children had adequate growth and all patients rated quality of life as exceeding 5 on the 10-point scale. Group 2 comprised patients similar to those in class 1, except for transfusion history: these patients had a longer history of transfusions. Group 3 comprised patients who had undergone splenectomy and in whom a beneficial response to splenectomy had been observed: in particular, during the 2 years following splenectomy, an improvement in quality of life and an increase in height velocity was observed. Group 4 comprised patients doing poorly without transfusions as shown by poor growth, delayed sexual maturation, and quality of life less than 5 on the 10-point scale. Group 5 comprised patients unable to function without transfusions.
| Class | Patient Group | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| No. | 25 | 37 | 14 | 22 | 11 |
| Age (y) | 4–51 (25.3 ± 16.5) | 9–52 (23.2 ± 10.6) | 5–16 (10.8 ± 3.0) | 5–21 (15.2 ± 3.4) | 4–13 (? ± 2.9) |
| Age diagnosis (y) | 1.5–4.8 (15.5 ± 13.4) | 0.8–35 (9.9 ± 8.4) | 0.4–56 (2.3 ± 2.1) | 0.7–8.0 (3.6 ± 2.6) | 0.2–4.0 (1.4 ± 0.8) |
| Maximum liver size (cm) | 0–14 (96.0 ± 3.5) | 3–18 (9.8 ± 3.5) | 6–16 (9.8 ± 1.6) | 5–19 (11.1 ± 3.8) | 0–12 (7.6 ± 1.7) |
| Maximum spleen size (cm) | 0–18 (8.9 ± 3.2) | 6–23 (13.4 ± 3.2) | 11–20 (17 ± 38) | 5–17 (11.4 ± 3.3) | 4–15 (9.9 ± 3.3) |
| Facial deformity (0–6) | 0–2 (0.9 ± 0.7) | 0–4 (1.5 ± 1.0) | 0–4 (2.0 ± 1.2) | 0.5–3.5 (1.92 ± 1.2) | 1–3 (1.5 ± 0.9) |
| Splenectomized (%) | 21.0 | 62.2 | 100 | 85 | 22 |
| Time from splenectomy (y) | 2–33 (13.3) | 2–19 (7.4) | 2–9 (3.1) | 1–13 (5.6) | 2–7 (4) |
| History major infection (%) | 8.3 | 18.9 | 16.7 | 26.3 | 11.0 |
| History of malaria (%) | 21 | 37.9 | 25.0 | 42.0 | 11.0 |
| Leg ulcers (%) | 33.0 | 51.3 | 16.7 | 15.8 | 0 |
| Fractures (%) | 0 | 13.9 | 0 | 25.0 | 56 |
| Gallstones (%) | 22.0 | 41.7 | 9.1 | 16.7 | 0 |
| Cholecystectomy (%) | 13.0 | 5.4 | 0 | 5.3 | 0 |
| Hb (g/L) | 31–82 (65 ± 12) | 36–83 (61 ± 10) | 42–71 (58 ± 10) | 44–80 (59 ± 12) | 40–58 (47 ± 7.0) |
| Before splenectomy | – | – | 3.5–4.3 (3.9) | – | – |
| After splenectomy | 6.5–7.6 (7.0) | 5.3–7.2 (6.4) | 6.4–8.7 (7.2) | 4.1–7.6 (6.1) | 4.0–5.5 (4.9 ± ?) |
| Platelets (10 9 /L) | |||||
| Before splenectomy | 157–898 (400.5) | 223–749 (432) | 203–576 (365) | 294–474 (392) | 287–503 (364.5) |
| After splenectomy | 379–918 (742) | 253–1051 (677) | 411–948 (690) | 303–974 (679) | 335–814 (574) |
| Hb F (g/L) | 5.0–38 (20 ± 12) | 2.0–37 (17 ± 7.0) | 5.0–25 14 ± 5.0) | 4.0–27 (15 ± 3.0) | 4.0–19 (9.0 ± 4.0) |
| Hb F (%) | 8–60 (32 ± 13) | 3–48 (29 ± 10) | 10–34 (20 ± 6) | 10–42 (26 ± 11) | 6–42 (17 ± 8) |
| β-Thalassemia Mutation (%) | |||||
| IVS-1-5 (G→C) | 52.0 | 73.5 | 74.6 | 59.1 | 75.0 |
| IVS-1-1 (G→A) | 39.0 | 26.5 | 27·3 | 22·7 | 13·0 |
| Others | 9·0 | 0 | 8·1 | 18·8 | 12·0 |
| α + -Thalassemia (%) | 8·0 | 8·1 | 7·1 | 4·5 | 6 |
| ααα/αα (%) | 4·7 | 3·0 | 0 | 0 | 0 |
| Xmn 1 +/+ (no.) | 8 | 5 | 0 | 0 | 0 |
| Xmn 1 −/− (no.) | 2 | 2 | 2 | 5 | 4 |
| ALT (U/L) | |||||
| HIC (mg iron per gram liver, dry weight) | 2.5–26 (9.3) | 2.7–42 (14.8) | 5.4–52 (16.7) | 4.7–30 (15.0) | 4.2–28 (16.3) |
Factors that influence the clinical severity of hemoglobin E/β-thalassemia
There is an increased understanding of the genetic and environmental factors that influence the clinical course and severity of anemia in HbE/β-thalassemia ( Box 1 ). These include the type of β-thalassemia mutation coinherited with HbE, the coinheritance of α-thalassemia, and the coinheritance of polymorphisms shown to be associated with increased synthesis of hemoglobin F (HbF).
Genetic Modifiers
β-Thalassemia mutation
Coinheritance of α-thalassemia
Mutations associated with increased HbF synthesis
XmnI polymorphism. Single nucleotide polymorphisms (SNPs) within the β gene cluster (chr.11p15). Quantitative trait loci (QTL) with increased F on chromosomes 6q23, 8q, and xp22
SNPs in the BCL11A gene on chromosome 2p16.1
Polymorphism of the UGT11 gene
Serum erythropoietin concentration
Environmental Modifiers
Malaria
Splenectomy
Inheritance of the β-Thalassemia Allele Trans to HbE Allele
In early studies Thai investigators suggested that patients who coinherit a mild β-thalassemia allele with HbE might have mild disease, whereas those who coinherited severe β + or β 0 -thalassemia alleles might have more severe disease. More recent studies suggest that the severity of the β mutation is an important, but uncommon, cause of the clinical diversity of HbE/β-thalassemia. In 1993, Winichagoon and colleagues studied the mutations in 90 Thai patients with steady-state hemoglobin concentrations varying from 4.2 to 12.6 g/dL. The same severe β-thalassemia mutation was observed in patients with mild as well as severe anemia. Thirty-six of these patients had a milder clinical phenotype. Nearly half (42%) coinherited a clinical modifying mutation. However, a less severe β-thalassemia mutation was only 1 of several genetic modifiers. Other modifiers included XmnI polymorphism, α + thalassemia, and hemoglobin H-Constant Spring.
A more detailed analysis of the effects of the β-thalassemia allele on clinical severity within this population was performed in 2000. A genotype responsible for a mild HbE/β-thalassemia phenotype was documented in 6 out of 88 patients. This mild phenotype resulted from the interaction between HbE and the mutation at nucleotide −28 in the ATA box of the β-globin gene. However, patients with mild disease usually had a severe β-thalassemia mutation. Studies in other HbE/β-thalassemia populations from India and Sri Lanka confirm that the β-thalassemia mutation plays a limited role in the clinical severity of this population. Therefore, the β-thalassemia mutation alone does not account for the wide phenotypic variation observed in this population, and other modifying genetic factors must be responsible.
α-Thalassemia
Patients with HbE/β-thalassemia who coinherit a determinant for α-thalassemia should, in theory, have fewer unmatched α-globin chains, leading to the more balanced globin chain synthesis, and resulting in a milder phenotype. Early studies suggested that patients with HbE/β-thalassemia with an α + -thalassemia allele averaged higher hemoglobins than those without α-thalassemia.
Recent studies support the beneficial effect of α-thalassemia on HbE/β-thalassemia but indicate that clinical severity is multifactorial. The effect of coinheritance of different copy numbers of the α-globin genes on the severity of HbE/β-thalassemia was analyzed in 925 Thai patients with HbE/β-thalassemia, aged 2 to 77 years, who showed a wide range of steady-state hemoglobin levels (3.2–12.1 g/dL); 8.8% of the study population had a form of α-thalassemia. Deletional α + -thalassemia was most often identified (−α 3.7 /αα (n = 51), −α 4.2 /αα (n = 8), and −α 3.7 /−α 3.7 (n = 1)). Two-thirds of the patients with normal α-globin gene numbers, and about a third (32%) required regular blood transfusions. By contrast, none of the patients with α-thalassemia required regular blood transfusions, although several had received 1 or more transfusions during intercurrent illnesses. The second group of patients with α-thalassemia was further divided into 6 subgroups according to the different α-thalassemia mutations identified. The mean age at presentation of those with α-thalassemia (10.4 ± 9.2 years) was much older than for patients without α-thalassemia (3.7 ± 5.5 years). The data suggested that patients with more severe α-thalassemia mutations may have had a better clinical picture.
Within a longitudinal study of patients with HbE/β-thalassemia in Sri Lanka, the influence of α-thalassemia in 5 groups of patients classified according to clinical severity was analyzed. The restriction of α + -thalassemia alleles to the groups with the mildest phenotypes suggests an ameliorating effect of α-thalassemia on the severity of HbE/β-thalassemia. However, there are conflicting reports, and mild HbE/β-thalassemia occurs without this mutation. In 148 patients in northeast Thailand (including 103 patients classified as severe and 45 as thalassemia intermedia), there was no evidence that coinheritance of α-thalassemia was a factor modifying the severity of HbE/β-thalassemia.
In summary, coinheritance with α-thalassemia seems to be a major genetic factor modifying the clinical phenotype of HbE/β-thalassemia. Many patients who coinherit α-thalassemia may be diagnosed later in life. The presence of α-thalassemia genes should always be considered in mild cases of HbE/β-thalassemia.
Determinants Associated with Increased HbF Synthesis
Several studies provide evidence supporting polymorphisms that affect HbF synthesis, including Xmn1 polymorphism, BCL11A, and several other genetic loci.
XmnI polymorphism is an important modifying factor in HbF synthesis in normals and in HbE/β-thalassemia. In Chinese β-thalassemia heterozygotes and in healthy Europeans, the Xmn1 polymorphism accounts for approximately 9% to 13% of the genetic variance of HbF. Thai patients with HbE/β-thalassemia and an Xmn1 +/+ genotype had higher total hemoglobin levels (8.5 g/dL vs <7.0 g/dL) and HbF levels than patients with an XmnI −/− genotype. These findings were consistent with previous findings suggesting that homozygosity for the Xmn1 polymorphism may be necessary to ameliorate the severity of thalassemia.
In a longitudinal study by Premawardhena and colleagues of patients with HbE/β-thalassemia in Sri Lanka, there was a strong positive correlation between the hemoglobin, fetal hemoglobin concentrations, and homozygosity for the Xmn1 polymorphism. Patients with the Xmn1 +/+ genotype were only found in the mild phenotypes. Patients with severe disease presenting at a young age were likely to have Xmn1 −/−. Others have reported that patients with Xmn1 −/− were more likely to present at a young age and be transfused compared with patients who were heterozygous (±). Other studies have analyzed the association of several SNPs within the β-globin gene cluster with disease severity. Although SNPs in the locus control region and the δ gene showed severity, the strongest association was with the Xmn1 polymorphism. Although there are some conflicting data, overall homozygosity for the Xmn1 polymorphism is one of the most important factors in modifying the severity of thalassemia.
Given the clinical heterogeneity of HbE/β-thalassemia, it is likely that additional genetic factors modifying disease severity await discovery. Association studies are being conducted to elucidate the role of genetic polymorphisms known to influence globin gene expression and erythropoiesis as modifiers of disease severity in this disorder. Other polymorphisms, QTL associated with increased fetal hemoglobin production, have been described on chromosomes 6q23, 8q, and Xp22. Recently, 6 SNPs in the BCL11A gene on chromosome 2p16.1 were reported to be associated with F cell numbers in a study of 179 unrelated normal subjects from a British twin registry, and with fetal hemoglobin levels among Sardinians with β-thalassemia. The contribution of the QTL on chromosome 6q23 to the genetic variance of HbF/F cells is estimated to be 19%, and the contribution of BCL11A to be 15%.
Sedgewick and colleagues reported that SNPs in BCL11A were associated with fetal hemoglobin production in Thais with either β-thalassemia or HbE trait (as well as in African Americans with sickle cell anemia) and with F cell numbers in Chinese individuals with β-thalassemia trait. Although the studies are limited, it is likely that BCL11a polymorphisms are also important modulators of fetal hemoglobin in HbE/β-thalassemia.
Sripichai and colleagues analyzed 1060 patients with HbE/β-thalassemia for 22 SNPs within 7 genes known to influence globin gene expression and erythropoiesis. These included β-protein 1, erythropoietin (EPO), and transcription factors EKLF, GATA-1, and NF-E2. No statistically significant difference between mild and severe phenotypes was observed for the polymorphisms in the candidate genes. A later genome-wide association study was conducted in 618 patients with HbE/β-thalassemia. Twenty-three SNPs in 3 independent genes/regions identified as being significantly associated with disease severity were studied. The highest association was observed with SNPs in the β-globin gene cluster (chr.11p15); rs2071348 of the HBBP1 gene. The next highest association was identified in the intergenic region between the HBS1L and MYB genes (chr.6q23). The third region was located in the BCL11A gene. The findings in these 3 loci were replicated in an independent cohort of 174 Indonesian patients. These data suggest that several genetic loci act in concert to influence fetal hemoglobin levels of patients with HbE/β-thalassemia, with these 3 reported loci and the α-globin gene locus identified as the best predictors of disease severity.
Other investigators have reported an association of the HBS1L-SNP7 polymorphism in exon 1 of the β-globin gene with HbF levels in 30 selected patients with mild and severe phenotypes of HbE/β-thalassemia, different levels of fetal hemoglobin, and different XmnI polymorphisms. Genotyping for a C32T polymorphism identified in a potential E-box binding site of HBS1L in exon 1 was then performed in 455 patients with HbE/β-thalassemia. HBS1L-SNP7 had a slight, but statistically significant, effect in modulating absolute fetal hemoglobin levels among patients with HbE/β-thalassemia and Xmn1 −/−. The effect of HBS1L-SNP7 was more pronounced among patients with Xmn1 ±. However, the overall conclusion was that this polymorphism is of minor effect in comparison with the influence of Xmn1 .
Other Modifying Factors
The α-hemoglobin stabilizing protein gene
The α-hemoglobin stabilizing protein, a chaperone of α-globin, has been suggested as another genetic modifier. However, it was not found to be a modifying factor of clinical severity in Thai patients with β-thalassemia. In patients with HbE/β-thalassemia in Sri Lanka, Premawardhena and colleagues found no difference in the distribution of haplotypes of this gene between patients with mild and severe phenotypes; this has been confirmed by other work.
Bilirubin metabolism
Chronic hyperbilirubinemia, gallstone formation, and gall bladder disease in patients with HbE/β-thalassemia may significantly worsen the phenotype of the disease. There is now clear evidence that the inherited variability in the function of the gene for UDP-glucuronosyltransferase-1 (UGT11, the enzyme responsible for hepatic glucuronidation of bilirubin) underlies the chronic hyperbilirubinemia of Gilbert syndrome, some cases of thalassemia trait, as well as the variation in the frequency of gallstones in patients with hereditary spherocytosis. The increased level of bilirubin in these disorders has been related to a polymorphism of the promoter of the UGT11gene.
Premawardhena and colleagues showed that the UGT1 genotype is also important in the genesis of gallstones in patients with HbE/β-thalassemia. He found significantly higher bilirubin levels in patients with HbE/β-thalassemia and the 7/7 genotype of the UGTA1A promoter, compared with those with 6/6 and 6/7 genotypes ( P = .032 and .0015, respectively). Patients with the 7/7 genotype were also more likely to develop gallstones at more than 15 years of age ( P ≥.0008).
Coinheritance of other hematologic disorders
Coinheritance of other hematologic abnormalities may contribute to the variation in phenotype in patients with HbE/β-thalassemia. Although pyrimidine 5 nucleotidase-I (P5N-I) deficiency typically presents as mild hemolytic anemia, a family in which the interaction between P5N-I deficiency and homozygous HbE resulted in severe hemolytic anemia has been reported. This individual was shown to have a mutation in the P5N-1 gene affecting expression of the P5N-1 enzyme, believed to be susceptible to free radical damage. Coinheritance of complete P5N-I deficiency and the homozygous HbE state results in a hemolytic phenotype that is more severe than would be expected with either condition alone, raising a possibility that coincidental inhibition of P5N-I activity may contribute to the severe hemolytic phenotype associated with some unstable hemoglobin variants, including HbE.
Variation in iron loading
A common dilemma often encountered in the clinic is whether the poor growth and delayed sexual maturation observed in many patients with HbE/β-thalassemia is a result of chronic anemia, iron overload, or a combination of these. As has been reported in a study in which precise measurements of iron loading were determined systematically, many patients with a clinical history of few transfusions have substantial iron burdens and evidence of end-organ damage that is not evident by screening tests. Further data on gastrointestinal iron overload in the Indian subcontinent and southeast Asia are still awaited. Although some studies have searched for the common hemochromatosis alleles in those regions and found them to be rare, Lok and colleagues recently reported several novel mutations in hemojuvelin and hepcidin, and mutations in the SLC40A1 gene, which encodes for ferroportin, in patients originating from countries not normally associated with hemochromatosis, resulting in different presentations of iron overload. These findings suggest that primary iron overload may not be as rare as was previously believed in these geographic areas. Studies to determine the effect of variability in iron loading on the phenotype of HbE/β-thalassemia are urgently needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree