A change in the colour of urine may be the earliest or sole clue that a surgical patient under general anaesthesia has received an ABO-incompatible unit, and even this sign may be masked by haematuria in the patient undergoing genitourinary procedures. Early recognition is essential – to prevent transfusion of additional incompatible blood and to avoid possible administration of a second unit to other than the intended recipient.
As described later in this chapter, episodes of intravascular haemolysis following the transfusion of incompatible red cells are sometimes followed by renal failure and by the production of various other untoward signs. In patients whose serum contains non-lytic or only very weakly lytic anti-A or anti-B, following the transfusion of ABO-incompatible blood the level of plasma Hb may be less than 1 g/l and no Hb is passed in the urine. In fact, only when anti-A and anti-B are relatively potent is destruction predominantly intravascular.
After the transfusion of incompatible blood, erythrophagocytosis may be observed in samples of peripheral blood (Hopkins 1910; Ottenberg 1911). Striking leucopenia may result from the adherence of leucocytes to red cells and to one another. In one investigation, after the injection of 10 ml of ABO-incompatible red cells, the total leucocyte count fell from 9000 to 4000 per microlitre in 5 min. The principal effect was on the mature granulocytes and monocytes. In further experiments, the injection of as little as 0.05 ml of incompatible red cells (5 × 108 cells) caused a significant fall in leucocytes. A calculated 15 leucocytes per injected incompatible red cell was removed. Infusion of the stroma of compatible cells produced no effect, but the infusion of the stroma of incompatible cells produced leucopenia. Incidentally, the injection of A substance into O subjects produced leucopenia in six out of seven instances (Jandl and Tomlinson 1958).
Haemolysis with a Unit from a Chimera
A 61-year-old male patient typed as blood group O received two units of group O red cells after elective kidney surgery. Immediately following transfusion, he developed evidence of intravascular haemolysis (Pruss et al. 2003). Serological re-examination revealed a mixed-field pattern of agglutination of red cells in one of the two transfused units. The donor of this unit proved to be a 24-year-old man with a twin sister. Both siblings showed an identical mixture of roughly 95% group O and 5% group B red cells and chimerism was confirmed by genotyping.
Red Cells Present as ‘Contaminants’
When the donor is ABO incompatible, acute haemolysis may be provoked by red cells present in transfused platelet preparations, granulocyte concentrates or in bone marrow and progenitor cell suspensions, even when these suspensions have been treated with a sedimenting agent to remove the bulk of the red cells (Dinsmore et al. 1983). In the latter circumstance, significant reactions may develop when the recipient has a high-titre isoagglutinin and unmanipulated incompatible grafts are infused rapidly, although significant and symptomatic haemolysis complicating ABO-incompatible transplantation is not usual after either marrow or PBSC infusion (Rowley et al. 2000).
Transplacental Haemorrhage
When the fetus is ABO incompatible, a large transplacental haemorrhage (TPH) may be associated with haemoglobinuria in the mother. In one such episode that followed an easy external cephalic version under general anaesthesia, the mother (group O) passed fetal Hb in the urine (0.2 g of Hb/dl). Four weeks later, a group A infant was born. The placenta showed a small area of separation. Of 14 further cases of external version, fetal cells were found in only one and in this case the mother’s blood had not been examined before version (Pollock 1968).
In another case, abruptio placentae followed a car accident after which the mother (group A) gave birth to a stillborn group B infant. The mother exhibited haemoglobinaemia and haemoglobinuria (Glasser et al. 1970). In one more case, the group O mother of a B infant developed haemoglobinuria, acute tubular necrosis and disseminated intravascular coagulation (DIC) (Samet and Bowman 1961).
Frequency of ABO-Incompatible Transfusions
Data regarding the frequency of ABO-incompatible red cell transfusions are hard to come by. Almost all surveys start with reported clinical episodes, such as haemoglobinuria following transfusion. For obvious reasons, such episodes are not always reported. Even then, the signs may be attributed to a cause other than the transfusion. Furthermore, transfusions of ABO-incompatible blood may not cause clinically obvious effects, particularly if the patient is anaesthetized. Therefore, the reported frequency with which ABO-incompatible blood is given must seriously underestimate the true frequency. Of course, the frequency with which the ‘wrong’ blood is given must be several-fold greater than that with which ABO-incompatible blood is given, as a random unit of blood will be ABO incompatible with a random patient about 36% of the time in Western nations (Linden et al. 1992).
Some estimates of the frequency of ABO-incompatible transfusion, based on the reporting of clinical episodes, are as follows: one in 10 000 units or one in 3000 patients (Wallace 1977); one in 18 000 transfusions, a figure based on almost half a million transfusions (Mayer 1982); one per 30 000 units transfused in a survey in which one-half of the participants relied on memory rather than written record (McClelland and Phillips 1994); and one in 33 000 transfusions (Linden et al. 1992). In an analysis of transfusion errors over a 10-year period in New York State (1990–99), Linden and co-workers (2000) documented erroneous blood administration as 1 in 19 000 red cell units, ABO-incompatible transfusions as 1 in 38 000, and acute haemolytic reactions or laboratory evidence of reactions as 1 in 76 000 red cell units transfused. This experience is similar to that of several national haemovigilance surveillance systems in Europe.
Another approach to discovering the incidence of giving the wrong blood is to start with records of blood issued by the laboratory and to discover from records to whom each unit was transfused. This may be described as the descending method of enquiry, contrasted with the ascending method already described. Of 2772 units, seven (0.25%) were transfused to unintended patients. Not a single one out of the seven had been reported, although at least three of the units were ABO incompatible (Baele et al. 1994). The estimate that one in 400 units is given to the wrong recipient may be atypically high; however, it does not include errors in labelling the sample taken from the patient or laboratory errors. Incredible as it sounds, the frequency of mislabelled and miscollected samples in an international survey of 62 hospitals (690 000 samples), was 1 out of every 165 samples, and in a subset of hospitals, miscollected samples containing the wrong blood in the tube occurred in 1 out of every 1986 samples (Dzik et al. 2003). These appalling findings (including the failures of reporting) emphasize that estimates that start from clinical episodes must be far too low.
It has long been possible to devise systems of checking the identity of units and patients that lead to a very low error rate. At the Mayo Clinic, blood bank personnel have total control of blood units from the time when they are withdrawn from the donor to the time when they are given to the patient, the ‘vein-to-vein’ principle. Blood bank nurse transfusionists administer almost all blood that is transfused to patients in their rooms, and monitor all transfusions administered in operating theatres (Taswell et al. 1981, supplemented by personal communication from HF Taswell). In the period 1964–73, in the course of transfusing 268 000 units of blood, there was only one occasion on which ABO-incompatible blood was known to have been transfused (Pineda et al. 1978a). Systems approaches and evolving technology, from the lowly identification bracelet to bar codes and bedside computers, have reduced labour and improved safety and efficiency (Davies et al. 2006). The presence of national patient identification systems in Sweden and Finland has been associated with rates of miscollected samples that were too low to estimate (Dzik et al. 2003).
Mortality Associated with ABO-Incompatible Transfusions
Statistics regarding frequency and mortality of early transfusions are incomplete and suspect. In the 25 years before frequent transfusion began in the Second World War, Kilduffe and DeBakey (1942) assembled a series of 43 284 transfusions with 80 haemolytic reactions (0.18%) and 32 deaths (0.07%) from haemolytic shock. An independent estimate from the same era quotes a mortality of 39 out of 19 275 for direct transfusions and 9 out of 8236 for transfusions of blood collected into citrate (Wiener and Moloney 1943). Some of these deaths may have resulted from volume overload rather than from acute haemolysis, so an accurate percentage cannot be calculated. Both approximations suffer at the very least from ‘positive event’ reporting bias. However, it is evident that from the time of these pre-modern era reports to the present, improved procedures, compatibility testing and positive identification methods have reduced dramatically the risk of acute haemolytic reactions and death.
Mortality Estimates in the Modern Era
Three later estimates of the mortality of ABO incompatible transfusions have been published: (1) in a hospital in the USA between 1960 and 1977, 0 out of 13; although three patients received 50 ml or less, five received 0.5 units or more, and five received 1 unit or more (sixth edition, p. 573); (2) in the State of New York in 1990–91, 3 out of 54 (Linden et al. 1992); and (3) in a series collected in London between 1940 and 1980, 0 out of 12; three patients received less than 50 ml, six received 200–600 ml, and three received 1–4 l (seventh edition, p. 649).
In December 1975, the USA FDA established mandatory reporting ‘when a complication of blood collection and transfusion is confirmed to be fatal’. The information collected between 1976 and 1978 was reviewed by Schmidt (1980a,b) who considered that in only 39 out of the 69 cases could transfusion be regarded as the primary cause of death. In 24 out of the 39, death was due to an incompatible transfusion; 22 out of the 24 were immediate reactions due to transfusion of ABO-incompatible blood, and the remaining two were DHTRs associated with anti-c or anti-E. Almost the same series of cases was reviewed by Honig and Bove (1980), in an analysis that stressed the errors that had led to the transfusion of incompatible blood. They concluded that out of 44 acute haemolytic reactions, 38 were due to ABO incompatibility. Both reviews emphasized that the commonest cause of ABO-incompatible transfusion is failure to identify the recipient correctly and that the commonest place where incompatible blood is transfused is the operating theatre.
Deaths reported to the FDA in the 10-year period from 1976 to 1985 (inclusive) were reviewed by Sazama (1990). Of the cases attributed to red cell incompatibility, 158 were due to acute haemolysis (ABO incompatibility in 131) and 26 to delayed haemolysis (mainly anti-c and anti-Jka). Mortality calculated from the number of units of red cells transfused during this decade approximates 1 in 250 000 transfusions, although deaths are almost certainly under-reported. The previously referenced 10-year study of transfusion errors in New York State reported five deaths, 4% of all patients with acute haemolytic transfusion reactions, for an average of 0.5 events per year or 1 per 1.8 million transfusions (Linden et al. 2000). Of the 212 fatalities reported to the US FDA in the years 2007–2011, 50 (23%) were related to haemolytic transfusion reactions, and gradually non-ABO fatalities have overtaken ABO-related deaths. (www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm302847.htm) Errors still remain a concern (4 of 9 fatalities in 2011), but the trends and the death rates in developing countries appear to be declining.
Factors Determining Outcome
Two important factors in determining the outcome of an ABO-incompatible transfusion are the potency of the anti-A (or anti-B) in the patient’s plasma and the volume of blood transfused. Rate of infusion may be a third. For example, in the case illustrated later in the text (see Figure 11.6) in which virtually no ill-effects from the transfusion of A blood to an O subject occurred, the anti-A agglutinin titre before transfusion was 32 and the antibody was only very faintly lytic in vitro. Few adverse effects were noted in 12 carefully monitored patients who received entire units of incompatible red cells intentionally, albeit slowly, as part of a preparatory regimen for ABO-incompatible bone marrow transplantation (Nussbaumer et al. 1995) (see below).
Although striking reactions may develop during the first few minutes of transfusion with incompatible blood, the clinical severity generally depends on the amount of blood transfused. Most fatalities have been associated with transfusions of 200 ml or more, and mortality approaches 44% for infusions exceeding 1000 ml. However, volumes as small as 30 ml have been implicated (Honig and Bove 1980). Small volumes of ABO-incompatible blood may pose a higher risk for children and adolescents. This belief, although intuitive, finds little support in the published literature. One author (HGK) is aware of a mistransfusion of approximately 25 ml of group A red cells that proved fatal to a 6-year-old, group O, 28-kg child.
Role of Disseminated Intravascular Coagulation
Transfusion of ABO-incompatible blood may prove lethal by precipitating a shock syndrome, initiating DIC or causing renal failure. In one series of 40 patients who received incompatible blood, four died as a direct result of the transfusion. This is the basis of the oft-quoted 10% mortality statistic. All four patients had been transfused during or immediately after major surgery. Severe and persistent hypotension was the main clinical manifestation. All four patients developed DIC; two died within 24 h from irreversible shock and two after about 4 days when evidence of acute uraemia appeared (Wallace 1977). The seriousness of DIC as a complication of ABO-incompatible transfusion is emphasized by an earlier series: of five patients transfused, who developed a haemorrhagic diathesis, all died (Binder et al. 1959). Nevertheless, in the majority of patients who receive ABO-incompatible blood, DIC is mild or undetectable and renal failure does not develop.
Destruction of Recipient’s Own Red Cells by Transfused Anti-A or Anti-B
On occasion, clinical signs of red cell destruction develop after the transfusion of group O blood or plasma to recipients of other ABO groups. As a rule, destruction is predominantly extravascular but, as these events are part of the spectrum of ABO haemolytic reactions, they will be discussed below.
Transfusion of Group O Blood to Recipients of Other ABO Groups
Levine and Mabee (1923) coined the term ‘dangerous universal donor’ to describe group O donors whose plasma contained agglutinins of high titre. Nevertheless, in the early years of the Second World War, group O blood was used for all emergency transfusions in the UK, usually without any crossmatching or other serological testing. Adverse reactions were rare. In a series in the USA in which patients of groups A, B or AB were transfused with group O blood the frequency of frank haemolytic reactions was 1% (Ebert and Emerson 1946). Experimental work on the transfusion of group O blood containing potent anti-A and anti-B to recipients of other groups has been described in the previous chapter. In the present section, a few examples will be given of accidental haemolytic transfusion reactions following the transfusion of ABO-incompatible plasma, either in the form of group O blood or as plasma alone.
The following case illustrates many of the features of a severe haemolytic transfusion reaction in a group A patient after the transfusion of blood from a ‘dangerous’ group O donor:
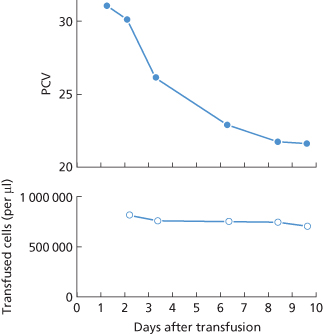
Figure 11.1 Destruction of recipient’s own (group A) red cells following the transfusion of group O blood, the plasma of which contained potent anti-A. The transfused cells survived normally and the progressive fall in the recipient’s packed cell volume (PCV) was therefore due entirely to the destruction of the patient’s own cells.
Plasma that has a comparatively low anti-A agglutinin titre (64–256) but contains potent IgG anti-A (IAT titre 1000–5000) may cause serious haemolytic reactions (Grove-Rasmussen et al. 1953; Stevens and Finch 1954).
Transfusion of Red Cells from Group O Blood to Recipients of Other ABO Groups
The injection of as little as 25 ml of plasma containing potent anti-A may produce haemoglobinuria in a group A subject. It is therefore not surprising that haemoglobinuria may occur after the transfusion of a unit of O red cells to a group A, B or AB subject. In a case in which the recipient was group A, the unit, which had a packed cell volume (PCV) of 0.70, contained estimated 95 ml of plasma. After ‘neutralization’ by group A- and B-specific substances, the plasma had an anti-A indirect antiglobulin test (IAT) titre of 8192. Apart from developing haemoglobinuria, the patient suffered a rigor and vomited, but made an uneventful recovery (Inwood and Zuliani 1978). In another case, a group B newborn infant with Escherichia coli sepsis developed fever and haemoglobinuria after exchange transfusion with red cells from group O blood suspended in AB plasma. The IgG anti-B titre of the group O donor was 64 000 and was not neutralized by AB substance (Boothe et al. 1993).
Transfusions of Group O Plasma from Single Donors
In three group A (or AB) infants, weighing 3.5–4.5 kg, who received 50–90 ml of group O plasma, jaundice developed within a few hours. Examination of films of peripheral blood showed striking microspherocytosis. In one case an increase in osmotic fragility was demonstrated. An interesting point in two cases was that plasma from the same donor had been given on the previous day without producing reactions. This suggested that the capacity of the body to inhibit anti-A had been saturated by the first plasma transfusion so that a second transfusion within a short time had a more damaging effect (Gasser 1945).
Renal failure has been described in a 3-year-old haemophiliac given a transfusion of 300 ml of group O plasma before dental treatment. The patient vomited before and after the subsequent anaesthetic. By the following morning he was deeply jaundiced and had passed a small amount of almost black urine. His Hb concentration was about 60 g/l and the DAT was strongly positive. The patient recovered after treatment with peritoneal dialysis. The transfused plasma proved to have a haemolysin titre of 128 and an agglutinin titre of 256 (Keidan et al. 1966).
Transfusion of Large Amounts of Low-Titre or Pooled Plasma
In some patients with burns, haemoglobinuria developed 12–48 h after transfusion of an amount of pooled plasma equivalent to more than three times their plasma volume. More than 40% of the recipient’s red cells were destroyed. The plasma used was prepared from the blood of 10 donors of different ABO groups (four group O, four group A and two group B or AB) and therefore had only low titres of anti-A and -B.
In a series of patients with haemophilia who were transfused with large amounts of pooled plasma, haemolytic episodes characterized by an elevated serum bilirubin concentration and a positive DAT were common. Pooled plasma frequently had anti-A titres of ‘immune’ (non-inhibited) antibody as high as 64 (Delmas-Marsalet et al. 1969).
Anti-A or Anti-B in Factor VIII Concentrates
A haemolytic reaction, characterized by a fall in the PCV to 0.20, microspherocytosis and haemoglobinaemia was caused by the administration of large amounts of a factor VIII concentrate to a group A patient (Rosati et al. 1970). In another group A patient, with a factor VIII inhibitor, who was given approximately 170 000 units of factor VIII, the DAT became positive, Hb fell to 50–60 g/l and the reticulocyte count rose to 54%; batches of the factor VIII preparation had anti-A IAT titres of 128–512 (Hach-Wunderle et al. 1989).
Anti-A and Anti-B in Immunoglobulin Preparations for Intravenous Use
Episodes of severe intravascular haemolysis were observed in two group B recipients of bone marrow transplants following the infusion of an immunoglobulin preparation (‘Gamimune’), later shown to contain anti-B with a titre of 32, as well as anti-A (Kim et al. 1988). Mild haemolysis or evidence of red cell sensitization without haemolysis is more common (Moscow et al. 1987).
Anti-A or Anti-B in Platelet Concentrates
A severe haemolytic reaction in a group A subject following the transfusion of 4 units of platelets from a group O donor suspended in 200 ml of the donor’s plasma was shown to be due to anti-A in the donor’s plasma with an IAT titre of 8192 and the ability to lyse A1 red cells. The recipient developed haemoglobinuria and the PCV fell from 0.22 before transfusion to 0.16 8 h later; the DAT became positive (Siber et al. 1982). Severe haemolysis is a particular risk when platelet concentrates are administered to infants and children.
The increasing use of single-donor platelets collected by apheresis and containing 200–300 ml of plasma has resulted in reports of haemolysis during ABO-incompatible transfusions, even with low-titre agglutinins (Mair and Benson 1998; Larsson et al. 2000; Josephson et al. 2004):
The frequency of such reactions is poorly documented. Larsson reported a frequency of 1 in 6600 when examining all single-donor platelet transfusions, whereas Mair and Benson (1998) found a haemolytic reaction frequency of 1 in 9000 when examining only plasma-incompatible transfusions. There is currently no standard for screening these units. The practice in the UK is to screen for high-titer apheresis platelets and only transfuse plasma-incompatible products if the titre lower than 50 or 100 (Sadani et al. 2006). At the NIH, a critical titre of 250 has been used as a practical compromise between risk and availability (Quillen et al. 2011); concentrates are not ordinarily screened for high-titre agglutinins.
Whereas virtually all reported cases of significant haemolysis involve transfusion of platelets from a group O donor, NIH encountered a group A donor with a high-titre anti-B that appeared to have been stimulated by repeated ingestion of a probiotic preparation and caused significant, symptomatic haemolysis in two recipients:
Whereas commercial probiotic preparations are known to contain bacteria that express blood group antigens, different preparations and indeed different lots from the same manufacturer vary widely in content and in viability of the organisms. A controlled study of volunteers who ingested the probiotic preparation reported in the case above resulted in several cases of abdominal distress and recurrent diarrhoea, but in no instances of significant elevation of haemagglutinin titres.
Haemolytic reactions from single donor platelets might be expected to decline or even disappear as platelets are increasingly stored in plasma-reduced platelet additive solutions. However haemolysis has been reported with ‘dry platelets,’ apheresis platelets resuspended in synthetic preservative media that contains no more than 25–30 ml of plasma (Valbonesi et al. 2000).
Destruction of Transfused A1 Red Cells by Passively Acquired Anti-A
In an A2 patient transfused with 5 units of A1 blood, followed by 1 unit of group O blood containing immune anti-A, all the A1 red cells were eliminated within about 2 days of transfusion and anti-A1 could be demonstrated in the recipient’s plasma (Ervin and Young 1950). In this case there was no evidence of adverse effects, but in another case in which a patient of subgroup A2 was transfused with three units of group A1 and 1 unit of group O blood, jaundice and anuria developed and the patient died (Grove-Rasmussen 1951).
Pathophysiology of Acute Haemolytic Reactions
Haemoglobin Release, Complement Activation and Liberation of Cytokines
Although clinical descriptions of acute haemolytic reactions were described in detail at the beginning of the twentieth century, and in fact as early as the seventeenth century (see below), the mechanisms remain incompletely understood. The reactions are triggered by the binding of antibody to the surface of the incompatible red cells, activation of complement and lysis of cells with release of Hb. Progression of the reaction then involves, to differing degrees, simultaneous activation of phagocytic cells, the coagulation cascade and the systemic inflammatory response involving both cellular and humoral components. The unpredictability of the response, the controlling factors of which remain maddeningly obscure, accounts for the differing signs and symptoms as well as the variable severity of these reactions.
Haemoglobin released into the bloodstream has cytotoxic and inflammatory effects (Wagener et al. 2001). Plasma haemoglobin has been associated with increased platelet adhesion and aggregation as well as vascular inflammation and obstruction in vivo (Simionatta et al. 1988). Nitric oxide binding by free haemoglobin may lead to smooth muscle dystonia with resultant hypertension, gastrointestinal contraction and vasoconstriction (Rother et al. 2005).
Activation of complement results in assembly of the membrane attack complex and liberation of the potent anaphylatoxins C3a and C5a, small polypeptides that increase vascular permeability, act directly on smooth muscle and interact with various cells. Complement fragments stimulate mast cells to degranulate and release vasoactive substances such as histamine and serotonin. Complement-coated cells bind to macrophages and other phagocytic cells, which, in turn, liberate interleukin 1 (IL-1) and other cytokines. Receptors for C3a and C5a are present on a wide variety of cells (leucocytes, macrophages, endothelial cells, smooth muscle cells), so that complement activation may be accompanied by production of free radicals and nitric oxide, release of leukotrienes and granule enzymes, and synthesis of interleukins (Butler et al. 1991; Davenport et al. 1994). C3a and C5a play a major role in producing the adverse effects of ABO-incompatible transfusions, but the release of cytokines may be equally important (Davenport 1995). Activation of the kallikrein system leads to further production of bradykinin with its powerful vascular effects (Capon and Goldfinger 1995).
In vitro, the incubation of A or B red cells with group O whole blood leads to the release of IL-8 and tissue necrosis factor (TNF) (Davenport et al. 1990, 1991). IL-8 has chemotactic and activating properties for neutrophils and is released by various cells, including monocytes. IL-8 production may be complement dependent (Davenport et al. 1990). TNF is one of the cytokines that plays a significant role in septic shock, a syndrome that shares many clinical characteristics with acute haemolytic reactions. TNF activates the intrinsic coagulation cascade and may thus take part in initiating DIC (Davenport et al. 1991, 1992). TNF not only causes endothelial cells to increase tissue factor production but also decreases production of thrombomodulin, which, in turn, suppresses protein C activity. Thus, coagulation is promoted and anticoagulation inhibited (Capon and Goldfinger 1995).
Hod et al. (2008) have established mouse models of haemolytic transfusion reactions. Transfusions of incompatible red cells IgG-mediated haemolysis characterized by rapid cell clearance, haemoglobinuria, elevated plasma levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6), and lower levels of TNF-alpha. These are the same proinflammatory cytokines implicated in the systemic inflammatory response syndrome (SIRS) and confirm the hypothesis that cytokine storm occurs as a result of haemolytic transfusion reactions. The same group has proposed that the ‘syndrome’ encompassed by these reactions may be explained as anaphylaxis provoked by an alternative, IgG-mediated pathway (Hod et al. 2009).
Symptoms and Signs Associated with Intravascular Haemolysis
The symptoms and signs associated with the transfusion of ABO-incompatible red cells were vividly described by Oehlecker (1928). As a biological test for compatibility, he infused 5–20 ml of blood from a potential donor and observed the effects. The following is a rough translation of what he noted when the donor was ABO incompatible:
Oehlecker pointed out that all symptoms usually subside rapidly, but that if more blood is infused the same symptoms recur within 1–2 min. The constricting pain in the chest may be due to a spasm of coronary vessels or to vascular occlusion by agglutinated cells.
Similar symptoms were noted in some of the early transfusions given to patients from animal donors. For example, the effects of transfusing calf blood to a human subject were reported as follows: after about 200 ml of blood had been transfused, the patient ‘found himself very hot along his arm and under the armpits’. After a second larger transfusion on the following day (of about 450 ml of blood) ‘he felt the like heat along his arm and under his armpits which he had felt before … and he complained of great pains in his kidneys and that he was not well in his stomach and that he was ready to choke unless they gave him his liberty’ (Denis 1667).
Towards the end of the nineteenth century, lambs were in vogue as donors and a certain Dr Champneys (1880) reported that he had witnessed more than a dozen transfusions given directly from the carotid vessels of a lamb into the forearm veins of human subjects suffering from phthisis. After a short interval subjects developed difficult breathing along with a feeling of oppression, then flushing, followed by sweating. On the next day and for a few days subsequently, ‘haematinuria’ and, in nearly all cases, urticaria were noted. Although constricting pain in the chest and pain in the lumbar region have been observed following the injection of 0.7 ml of A1 red cells to a subject whose serum contained potent lytic anti-A (fifth edition, p. 549), the intravascular lysis of approximately 1 ml of ABO-incompatible red cells does not necessarily cause symptoms (tenth edition, p. 365). Similarly, when 10 ml of washed ABO-incompatible red cells were injected, only a few subjects developed transient symptoms: lumbar pain, dyspnoea, hyperperistalsis, flushing of the face and neck (Jandl and Tomlinson 1958). Several millilitres of incompatible cells are infused routinely with out-of-group allogeneic stem cell grafts, and are usually well tolerated (Braine et al. 1982; Dinsmore et al. 1983). These patients frequently undergo extensive plasmapheresis to reduce the antibody titre and receive heavy premedication, including corticosteroids, prior to the infusions. However, 12 patients received deliberately mismatched donor-type red cell transfusions (1 unit over 8 h on each of two consecutive days) to adsorb isoagglutinins from the recipient. The units were well tolerated, although one patient with pre-existing impaired renal function developed reversible renal failure after the stem cell transplant. Recipient antibody titre ranged from 32 to 1024 (Nussbaumer et al. 1995). Nevertheless, the experience with a relatively few cases may owe as much to good fortune as to low risk, and this procedure has not received wide acceptance (Davenport 1995).
In another series of experiments, an immediate reaction occurred only when intravascular red cell destruction occurred. In this series, 51Cr-labelled group B red cells were injected into a group A patient with hypogammaglobulinaemia whose serum contained no detectable anti-B. The B red cells initially underwent only slow destruction corresponding to a T50Cr of about 5 days. After 5 days, various amounts of anti-B were injected, and only when the amount of anti-B was sufficient to produce intravascular lysis were any symptoms noted; facial flushing began 2 min after injection, lasted 4 or 5 min, and was followed 7 min after injection by pain in the groin, thighs and lower back lasting 45 min. Subjects receiving small injections of red cells of incompatible ABO groups developed a small rise in body temperature but no chills (Jandl and Kaplan 1960).
Strongly lytic antibodies, other than anti-A and anti-B, which produce similar effects in vivo are rare. In the first subject in whom anti-PP1Pk (-Tja) was identified, a test injection of 25 ml of incompatible blood was followed by an immediate severe reaction with haemoglobinaemia (Levine et al. 1951). In a subject with haemolytic anti-Vel, transfusion produced a rigor, lumbar pain and anuria (Levine et al. 1961).
As noted earlier, signs and symptoms of acute haemolysis may vary. The ‘classic’ presentation is a dramatic, life-threatening syndrome characterized by a feeling of dread, flushing, fever and chills, pain at the infusion site, lumbar spine and flanks, nausea, vomiting and shock. Patients may hyperventilate and develop cyanosis, or chest and abdominal pain may predominate, possibly as a result of occlusion and ischaemia in the microvasculature. Dyspnoea is common and the lung is an early, important, perhaps underappreciated target organ. Goldfinger and co-workers (1985) had the opportunity to observe a patient who received 20 ml of ABO-incompatible blood at the time of intensive haemodynamic monitoring. The earliest event, an increase in pulmonary vascular resistance, occurred within the circulation of the lung. Some patients will have minimal symptoms, and the first clues may be pallor, icterus and a fall in circulating Hb.
In patients who are anaesthetized or are heavily medicated during transfusion, the two signs that may call attention to the possibility that incompatible blood has been transfused are hypotension, despite apparently adequate replacement of blood, and abnormal bleeding.
Disseminated Intravascular Coagulation Associated with Intravascular Haemolysis
In dogs, the infusion of autologous haemolysed red cells leads to intravascular coagulation (Rabiner and Friedman 1968). Thromboplastic substances in stroma appear to be responsible. In monkeys, the infusion of sonicated stroma, free of Hb, produces a fall in platelets, fibrinogen and factors II, V and VIII (Birndorf et al. 1971).
The fully developed syndrome of DIC is characterized by thrombocytopenia, a fall in the levels of factors V and VIII (particularly the latter), a low level of fibrinogen and deposition of fibrin thrombi in small vessels. Fibrin degradation products can be demonstrated in the serum.
In experiments in monkeys in which incompatible plasma was transfused, evidence of mild DIC was observed in two animals in which plasma Hb levels reached 6 g/l or more. No bleeding was observed in animals in which the plasma Hb level was below 1 g/l (Lopas et al. 1971). In other experiments in which incompatible allogeneic red cells were transfused, equivalent in amount to 250 ml of blood to a human adult, DIC was observed in three out of five cases in which intravascular haemolysis (average plasma Hb 6.5 g/l) associated with haemolytic antibodies. The main features were a fall in factors V, VIII and IX and a less consistent fall in fibrinogen concentration and in platelet count. Intravascular coagulation was not observed in two cases in which destruction was predominantly extravascular and associated with predominantly incomplete non-haemolytic antibodies; in these latter cases there was a slow rise of plasma Hb, reaching a peak of about 0.5 g/l in 4 h (Lopas and Birndorf 1971).
Following the transfusion of ABO-incompatible blood, a haemorrhagic state may develop after as little as 100 ml. If the patient is undergoing operation, uncontrollable bleeding from the wound develops; epistaxis and bleeding from the site of venepuncture have also been observed. A fibrinogen level as low as 15 mg/dl has been reported, with virtually incoagulable blood. Fibrin degradation products (FDPs) have been found in the serum, usually in concentrations in the range of 250 to 450 µg/ml, but as high as 1900 μg/ml in one case (Sack and Nefa 1970).
Various interactions have been demonstrated between the complement, kinin, coagulation and fibrinolytic systems (Kaplan et al. 2002). For example, low molecular-weight fragments of factor XIIa (Hageman factor) may mediate C1 activity (Ghebrehiwet et al. 1981). The possible role of cytokines in precipitating DIC has been discussed above.
After predominantly extravascular destruction, DIC occurs only very occasionally. There are two reports in the literature of abnormal bleeding following incompatible transfusion due to antibodies that are not haemolytic in vitro: one due to anti-c (Wiener 1954) and one due to anti-Fya (Rock et al. 1969).
Renal Failure Following Intravascular Haemolysis
Destruction of red cells within the bloodstream liberates Hb into the circulation and is sometimes followed by a decline in renal function The postulated direct nephrotoxicity of Hb itself, although widely taught, has not been unequivocally demonstrated, remains hotly debated (Viele et al. 1997) and probably does not exist outside of the vasoconstriction mediated by nitric oxide binding (see below). Of 41 cases of renal failure related to haemolytic reactions, 21 were related to ABO incompatibility (Bluemle 1965). Almost all instances of renal failure associated with ABO incompatibility occur in group O patients, although only about 70% of ABO mismatched transfusions involve group O patients (Doberneck et al. 1964; Bluemle 1965).
The infusion of large volumes of stroma-free Hb has no effect on renal function in dogs and monkeys (Rabiner et al. 1970; Birndorf et al. 1971). On the other hand, when 250 ml of a preparation of Hb containing only 1.2% stromal lipid was administered at the rate of 4 ml/min to six well-hydrated healthy men, urinary output fell by 81%, mean creatinine clearance declined, and transient bradycardia and hypertension developed (Savitsky et al. 1978). In several studies, the infusion of Hb has been associated with vasoconstrictor effects such as increased blood pressure and reduced cardiac output (Hess et al. 1993; Thompson et al. 1994). Vasoconstriction may be produced by the interference of Hb with the action of nitric oxide. Hb is presumed to leak across the endothelial layer into the extravascular space. Hb is thought to interfere with nitric oxide function as nitric oxide diffuses from endothelial cells to smooth muscle, where it exerts its vasodilatory effects by regulating smooth muscle tone (Patel and Gladwin 2004). Hb breakdown products, for example metHb, may also play a role in generating damaging oxygen radicals (see also Ogden and MacDonald 1995).
Renal damage associated with intravascular destruction is relatively common when destruction is caused by potent lytic antibodies, and unusual when caused by non-lytic antibodies. Consumption coagulopathy with microvasculature clot deposition may compromise the function of several organs. In experiments in monkeys, in which haemolytic transfusion reactions were produced, fibrin thrombi were widespread and in at least one case were found in renal tufts (Lopas et al. 1971). Hypotension is doubtless another factor in precipitating renal failure. Potent complement activation leads to the release of large amounts of C3a and C5a, which, in turn, release vasoactive peptides from mast cells.
Renal Failure Following the Infusion of Stroma
There is evidence that the infusion of stroma from incompatible red cells is followed by renal failure. In one case, in an attempt to depress the titre of a panagglutinin in a patient’s plasma, stroma from four units of red cells was infused. One hour later, the patient felt apprehensive and sustained a fall in blood pressure, a rapid decrease in urinary output, granulocytopenia and thrombocytopenia. Following an infusion of mannitol (25%), urinary flow was rapidly restored. In a second case, a patient whose serum contained anti-K was infused with stroma from 1 unit of K-positive red cells and three units of K-negative red cells. There followed a rigor and severe oliguria lasting 5 days. The authors stated that in 12 other cases in which stroma had been infused, no complications occurred (Schmidt and Holland 1967). These experiments reinforce the conclusion that transfusion of incompatible red cells produces its damaging effect on the kidney not by releasing Hb but by activating complement, liberating cytokines and triggering DIC.
Effect of Incompatible Transfusion in an Anuric Patient
A patient of group O with presumed tubular necrosis following severe injury 1 month earlier was given one-third of a unit of group AB blood over 4 min. The patient felt unwell and temporarily lost consciousness. At 1 h, plasma Hb had risen to 4.8 g/l, 1.82 g bound to haptoglobin (Hp) and the rest free. The plasma was cleared of Hb in about 10 h, during which time the patient excreted 8 ml of faintly orange urine. The episode may have delayed slightly the onset of diuresis, but ultimately renal function was only slightly impaired (Hoffsten and Chaplin 1969).
Management of Suspected Acute Haemolytic Transfusion Reactions with Intravascular Haemolysis
Methods of diagnosing acute haemolytic transfusion reactions and the immediate steps to be taken when acute haemolysis is suspected are described later in this chapter; the serological investigations to be undertaken are discussed in Chapter 8.
Management of acute haemolytic reactions is both expectant and supportive. Early recognition and interdiction of further incompatible blood may be the single most important step. Although several treatment protocols have been proposed, there is no evidence that the supportive measures should differ from those administered for shock, renal failure and DIC from any cause. The degree of intensive medical support will depend on the severity of the reactions.
Hypotension is usually managed by aggressive fluid resuscitation, and when pressors are indicated, drugs that preserve renal blood flow, for example dopamine infused at 3–5 μg/kg per minute, are preferred. Timely intervention may limit the degree of renal impairment. Both prophylaxis and immediate treatment of renal insufficiency traditionally include mannitol 20% (100 ml/m2) and diuretics to maintain a minimal urinary output of 0.5 ml/kg per hour, but little scientific evidence supports this recommendation. Further management depends on the clinical response. Alkalinization of the urine is routinely recommended, may be helpful, and is unlikely to cause harm.
Appropriate management of the consumption coagulopathy in acute haemolytic transfusion reactions, as in other conditions, is fiercely debated. There is no evidence that prophylactic anticoagulation prevents or lessens the microvascular thrombosis or the bleeding. Heparin administration has been advocated by some once the diagnosis of DIC has been established, and if prescribed, a dose of 5000 units immediately, followed by a continuous infusion of 1500 units/hour for 6–24 h has been recommended (Goldfinger 1977). Heparin treatment carries the risk of exacerbating the bleeding. Few reports address the role of heparin during acute haemolytic transfusion reactions, although two apparent successes were reported by Rock and co-workers (1969). Use of blood components such as plasma, platelets and cryoprecipitate are equally controversial, but should in any case be limited to patients with life-threatening bleeding. As acute haemolytic transfusion reactions so often result from a transfusion error, special effort must be undertaken to ensure that any blood component selected has been determined to be compatible.
Non-Immunological Intravascular Haemolysis
Haemolysis Due to Osmotic Effects
Damage Produced by Exposure to 5% Dextrose
Haemolytic transfusion reactions have been observed in patients receiving transfusions of whole blood passed through a bottle containing 5% dextrose and 0.225% saline [Ebaugh et al. 1958, cited by DeCesare and co-workers (1964)]. Similarly, red cells suspended in excess 5% dextrose or in 4.3% dextrose with 0.18% saline were almost completely lysed after 24 h at room temperature (Noble and Abbott 1959). On the other hand, when blood was mixed with 5% dextrose in normal saline, no lysis was found even after 15 h at 37°C (Ryden and Oberman 1975). The small number of agglutinates with negligible haemolysis that is sometimes observed in the intravenous tubing when a 5% dextrose solution follows transfusion of red cells is rapidly diluted in the patient’s circulation and has not been associated with adverse events.
Injection of Water Into the Circulation: Best Be a Rabbit
Infusion of 100 ml of water into an adult produces only slight haemoglobinaemia (0.1–0.2 g/l), but the injection of 300–900 ml, infused over a period of 1–4 h, produces plasma Hb levels of 2–4 g/l. Water may gain entrance to the circulation if the bladder is irrigated with water rather than with saline during transurethral prostatectomy (TURP). Acute renal failure after TURP may be caused by hypotonicity and hypervolaemia with subsequent increased vascular leakage leading to hypotension and rapidly impaired renal function. Acute renal failure caused by haemolysis after TURP has been reported, but is rare (Gravenstein 1997). In patients who already have renal ischaemia, the entry of water into the circulation may precipitate renal failure (Landsteiner and Finch 1947).
Injection of water into the circulation can be lethal. Two patients who were accidentally given 1.5 and 2 l, respectively, of distilled water by rapid infusion developed rigors, haemoglobinuria and persistent hypotension, and both patients became oliguric and died (J Wallace, personal communication). Despite the evidence that infusion of large amounts of water is dangerous in humans, very large amounts have been given to rabbits without producing ill effects (Bayliss 1920).
Insufficiently Deglycerolized Red Cells
Osmotic stress may cause lysis of red cells that have been cryopreserved with glycerol but inadequately washed free of the cryoprotectant after thaw. In most cases, haemolysis is minimal and patients may note a change in urine colour, but no other signs or symptoms.
Clinical symptoms of a haemolytic reaction occurred in one of two patients with sickle cell anaemia after in vivo haemolysis of transfused deglycerolized red cells (Bechdolt et al. 1986). Instrumentation is available that controls automated deglycerolization and should reduce this risk.
Transfusion of Haemolysed Blood
If enough free Hb is injected into the circulation the resultant haemoglobinaemia may be misinterpreted as a sign of intravascular haemolysis. Appreciable quantities of free Hb may be injected in any of the following circumstances.
Transfusion of Overheated Blood
Red cells are damaged and destroyed if warmed to a temperature of 50°C or more. Most heat-related accidents happen when a unit of blood is placed in a vessel containing hot water with the intention of warming the blood to body temperature. The transfusion of approximately 2.5 l of blood that had been accidentally overheated, and haemolysed, was associated with irreversible renal failure and death of the patient (J Wallace, personal communication). One of the authors (HGK) is aware of a similar tragic occurrence following transfusion of a unit of red cells that had been ‘warmed like a baked potato’ in a commercial microwave oven. As discussed in Chapter 15, blood should not be warmed before transfusion except in special circumstances and using methods in which the temperature is carefully monitored.
Transfusion of Blood Haemolysed by Accidental Freezing
Blood may be accidentally frozen, either by storage in an unmonitored refrigerator or by inadvertent placement in a freezer. In one reported case, three units of autologous blood, donated 2–4 weeks previously, had been accidentally frozen at −20°C and were transfused during hip surgery. Six hours later, the patient’s urine was noted to be dark red and oliguria developed. There were no signs of shock. The patient required repeated haemodialysis, but eventually recovered (Lanore et al. 1989).
Blood Forced Through a Narrow Orifice: Easier to Pass Through the Eye of a Needle
Forcing blood through a narrow opening such as a narrow-gauge needle has been incriminated as the cause of haemolysis in several different circumstances:
Transfusion of Infected Blood – and of Infected Patients
Blood that has been contaminated with certain bacteria and stored may become grossly haemolysed. The transfusion of such blood does produce haemoglobinaemia and haemoglobinuria, but these signs pale into insignificance when compared with the very toxic effects of bacterial toxins (see Chapter 16). Haemolysis due to bacterial sepsis may mimic a haemolytic transfusion reaction (Felix and Davey 1987).
Transfusion of Red Cells with Intrinsic Defects – and of Patients with Red Cell Defects
Transfusion of cells with certain enzyme or membrane defects may result in acute haemolytic transfusion reactions, although in most instances haemolysis is asymptomatic, delayed or appreciated only in retrospect by a shortened interval between transfusions. Exchange transfusion with G6PD-deficient blood has resulted in acute haemolysis in infants (Kumar et al. 1994). Immediate post-transfusion haemolysis was documented in 6 out of 10 Israeli adults who received G6PD-deficient blood, although the degree of red cell destruction was minimal and no symptoms were noted (Shalev et al. 1993). The risk and degree of haemolysis depend on the particular enzyme variant and may be exacerbated by the concurrent administration of medications associated with oxidative stress (Beutler 1996; see also Chapter 1). Acute haemolysis related to enzyme deficiencies may mimic acute haemolytic transfusion reactions, particularly in the surgical setting (Sazama et al. 1980). Units from donors with hereditary spherocytosis and poikilocytosis may also result in haemolysis either during storage or post transfusion (Weinstein et al. 1997).
Autoimmune Haemolytic Anaemia
Haemoglobinuria following transfusion in autoimmune haemolytic anaemia (AIHA) seems most likely to be caused by increasing the red cell mass subject to autoimmune destruction in patients with a very severe haemolytic process (Chaplin 1979). Haemoglobinuria following transfusion in patients with complement-mediated AIHA may be due to the initial destruction of ‘unprotected’ red cells that, unlike the patient’s own red cells, have little C3dg on them. Intravascular lysis due to the increased supply of complement in the transfused blood is probably an unusual cause of post-transfusion haemoglobinuria in patients with AIHA; however two possible cases, both in patients with cold haemagglutinin disease, were described by Evans and co-workers (1965).
Paroxysmal Nocturnal Haemoglobinuria: Dispelling the Washing Myth
Normal red cell membranes express glycosyl phosphatidylinositol-anchored proteins (GPI-AP), two of which, decay accelerating protein (DAF or CD55) and membrane inhibitor of reactive lysis (MIRL or CD59), regulate the complement cascade (Brodsky 2008). Patients whose haematopoietic progenitor cells develop clones containing a mutant X-linked phosphatidylinositol glycan complementation class A (PIGA) gene do not express a normal glycosyl transferase (or normal GPI-AP) and lack the two complement regulatory proteins; their red cells are variably sensitive to complement-mediated lysis (Parker 2007). The diagnosis can be made by flow cytometry (Richards et al. 2000). In some cases, the great majority of red cells are derived from the mutant clone, whereas in others, 10% or fewer circulating cells lack GPI-AP. Furthermore, the cells may vary in the degree of GPI-AP (Parker 2007). These quantitative and qualitative disparities among the red cells explain in part the clinical variability of the rare disorder known as paroxysmal nocturnal haemoglobinuria (PNH) (Parker et al. 2005). PNH type III cells are the most sensitive to complement mediated lysis.
PNH is characterized by haemolysis, thrombosis, and bone marrow insufficiency. Management includes the use of red cell transfusion, which, along with the haemolysis of the sensitive cells, may lead the unwary to underestimating the size of the PNH clone. Although nocturnal haemoglobinuria occurs in only about a quarter of patients (Dacie and Lewis 1972), patients with PNH may develop Coombs-negative intravascular haemolysis and haemoglobinuria after transfusion. The postulated mechanism involves activation of the complement cascade to which PNH red cells are abnormally sensitive. Customary practice for transfusing PNH patients has been to wash the red cells free of plasma in an effort to minimize complement and alloantibodies in the transfusion. However, in a 38-year experience at the Mayo Clinic, 23 patients with PNH were transfused with 556 blood components that included 94 units of whole blood, 208 units of red cells, 80 units of white cell-poor red cells, 38 units of washed red cells, five units of frozen red cells and 6 units of red cells salvaged during surgery. Only one documented episode of post-transfusion haemolysis related to PNH was confirmed. That episode was associated with the transfusion of a unit of group O whole blood with ABO-incompatible plasma to an AB-positive patient and probably involved antibody-mediated complement fixation on the red cell membrane (Brecher and Taswell 1989). This analysis suggests that the routine use of washed cells for PNH patients is unnecessary as long as ABO-identical blood components are transfused. But complement can be activated by antigen–antibody reactions not localized to the susceptible red cell membrane. Sporadic reports do suggest that leucocyte antibodies in transfused components may precipitate haemolysis in PNH recipients (Sirchia et al. 1970; Zupanska et al. 1999). If such antibodies are present, prudence dictates washing the cells free of plasma.
A recombinant humanized monoclonal antibody (eculizumab), developed originally to treat autoimmune disease, inhibits activation of terminal complement components and has been shown in a randomized trial to reduce haemolysis and transfusion requirement in patients with chronic haemolysis of type III PNH cells (Hillmen et al. 2006). Several years’ experience with this drug confirm its efficacy in decreasing haemolysis, and perhaps in improving thrombocytopenia and reducing the risk of thrombosis as well (Hillmen et al. 2007); the fear that an expanded cohort of complement-sensitive cells might result in fulminant haemolysis should the drug be discontinued has fortunately as yet not been realized.
Sickle-Cell (SS) Disease and Sickle Cell Haemolytic Transfusion Reaction Syndrome
Accelerated haemolysis (hyperhaemolysis) may occur during acute vasoocclusive episodes, although the magnitude of the increase has been debated and the mechanism has been attributed localized vascular changes at the site of necrosis (Neely et al. 1969; Naumann et al. 1971). Accelerated haemolysis also occurs in some patients with SS doubly heterozygous for G6PD deficiency , or infected with malarial parasites, Babesia microti, and Mycoplasma pneumoniae. More usually, ‘hyperhaemolysis’ in patients with SS disease refers to the rapid destruction of transfused red cells (and autologous bystander cells) consistent with either acute or delayed immunologic reactions. The classic description of this syndrome emphasized the presence of typical SS crisis pain and rapid destruction of large volumes of transfused cells despite compatible crossmatch results (Chaplin and Cassell 1962; Diamond et al. 1980). Symptoms suggestive of a sickle cell pain crisis develop or are intensified during the haemolytic reaction and may result in more severe anaemia after the transfusion than was present originally. Patients may have either marked reticulocytosis or severe reticulocytopenia. The syndrome(s) have been referred to collectively as the sickle cell haemolytic transfusion reaction syndrome (Petz et al. 1997). The pathophysiology of the hyperhaemolysis that appears to destroy autologous as well as transfused cells is not predictable (Petz et al. 1997), and the appropriate clinical management of this life-threatening complication has yet to be established (Telen 2001). Immune-mediated haemolysis is not always demonstrable when hyperhaemolysis occurs in the setting of recent transfusion (Aygun et al. 2002). Although hyperhaemolysis has most frequently been associated with sickle cell disease, life-threatening haemolysis has also been reported in patients with thalassaemia and in patients without any recognized haemoglobinopathy (Darabbi and Dzik 2005) (see also Chapter 11).
Bleeding Into Soft Tissues
Massive but occult bleeding into soft tissues, typically retroperitoneal bleeding or haemorrhage into the thigh after femoral artery puncture, may mimic acute or DHTRs. Rapid fall in Hb concentration may be followed by elevation in serum bilirubin, lactate dehydrogenase and fibrin degradation products as clot is resorbed (HG Klein, personal observation).
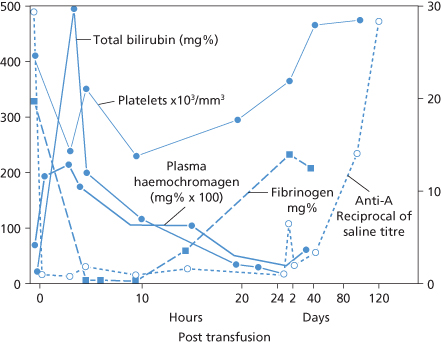
Clearance of Haemoglobin from the Plasma (Figure 11.2)
Haemoglobin liberated into the plasma dissociates into dimers that bind to haptoglobin (Hp) (see Bunn et al. 1969). Unbound Hb is partly processed by the liver and partly excreted (as dimers) in the urine. Free Hb is readily oxidized in the plasma to metHb; after dissociation from globin, haem binds preferentially to haemopexin. The globin split off from Hb is bound by Hp. Hb catabolism and other laboratory measurements during a representative acute haemolytic transfusion reaction are displayed in Figure 11.2 (Duvall et al. 1974).
Figure 11.2 Time course of changes in anti-A titre, plasma Hb (mg/100 ml × 10), total bilirubin (mg/100 ml), platelets and fibrinogen following transfusion of 140 ml of incompatible (group A2) red blood cells to a group O patient.
(Source: Adapted from Duvall 1974. Reproduced with permission of John Wiley & Sons Ltd.)
Haptoglobin (Hp) is a normal plasma protein, capable of binding about 1.0 g of Hb per litre of plasma. When amounts of Hb not exceeding this level are infused or liberated into the bloodstream, the Hb circulates as a complex with Hp. The molecular weight of Hp varies according to phenotype, and is about 100 kDa for Hp 1–1 and about 220 kDa for Hp 2–1 (Giblett 1969). Molecules of Hp tend to bind with dimers of Hb rather than with whole molecules (tetramers) of Hb to give a complex with a molecular weight (for Hp 1–1) of 135 kDa. When Hb tetramers are bound, the complex has a molecular weight (for Hp 1–1) of 169 kDa.
The complex Hb–Hp is taken up by hepatic parenchymal cells. When the amount of Hb liberated into the plasma corresponds to only a few grams per litre, the Hb complex is cleared exponentially with a half-time on the order of 20 min (Garby and Noyes 1959), but at higher Hb levels the clearance system is saturated and a constant amount of approximately 0.13 g of Hb per litre of plasma is cleared per hour (Laurell and Nyman 1957; Faulstick et al. 1962). After the injection of amounts of Hb calculated to suppress the Hp level to zero, the Hp level rose to 50% of the pre-injection level in 36 h and to 100% in 7–9 days (Noyes and Garby 1967).
Haemopexin (Hx) is a protein present in plasma in low concentration, to which methaem, derived from circulating free metHb, binds preferentially. Hx also binds haem from methaemalbumin and may provide the primary clearance route for haem complexed in this way (Muller-Eberhard et al. 1969).
Methaemalbumin is a pigment formed by haem (methaem) that is not bound to Hx, bound with albumin. Apart from acute haemolytic incidents, methaemalbumin is found in the plasma only when the amount of Hp has been reduced to a negligible level, less than 5 mg/dl (Nyman et al. 1959). The rapid infusion of 14 g of Hb into an adult will lead to the formation of sufficient methaemalbumin to give a positive Schumm’s test, although about three times this amount of pigment must be present before it can be detected spectroscopically. Methaemalbumin can be detected about 5 h after injecting Hb and remains detectable for 24 h or more (Fairley 1940).
Haemoglobinuria
When the amount of Hb not bound by Hp reaches about 0.25 g/l, some is excreted in the urine. The clearance of Hb by the kidney is only 5% of that of water or 6 ml of plasma per minute per 1.73 m2 of body surface compared with 100 ml plasma per minute for insulin (Lathem 1959). Some Hb is reabsorbed by the renal tubules (Lathem et al. 1960). At plasma Hb levels of approximately 1.8 g/l induced in normal males, the amount of Hb reabsorbed was approximately 1.4 mg/min, which was about one-third of the amount being filtered by the glomeruli (Lowenstein et al. 1961).
The fact that in most subjects haemoglobinuria occurs only when the plasma level exceeds about 1.5 g/l was known long before the role of Hp was appreciated (Ottenberg and Fox 1938; Gilligan et al. 1941). The latter investigators found that when the initial plasma Hb concentration was 0.4–0.6 g/l, the plasma was cleared in 5 h; when the initial level was 1.0–2.25 g/l the period was 8 h; and when the initial level was 2.8–3.0 g/l the plasma was not cleared for 12 h.
After the infusion or liberation of relatively large amounts of Hb into the circulation, up to one-third may be excreted in the urine. In six subjects receiving rapid injections of 12–18 g of Hb, the average amount excreted was about 18% of the amount injected (Amberson et al. 1949). In dogs transfused with incompatible blood in amounts equivalent to giving 200–1000 ml to an adult human, the amount of Hb excreted in the urine varied from 10% to 40% of the amount in the transfused red cells (Yuile et al. 1949).
Haemosiderinuria
When free Hb is filtered through the glomeruli, some or all of it is reabsorbed by the renal tubules and the iron released is stored as haemosiderin. If this process continues for a long period, iron-laden cells and free haemosiderin are found in the urine (see Bothwell and Finch 1962, p. 413). Haemosiderinuria is invariably found in adults whose plasma Hb concentration exceeds 0.25 g/l. Only microscopic amounts are found when the plasma Hb is below 0.20 g/l, but larger amounts are found when the level exceeds 0.50 g/l (Crosby and Dameshek 1951).
Bilirubinaemia Following Infusions of Haemoglobin
The serum bilirubin concentration rises by about 0.5 mg/dl (1 mg/dl = 17 µmol/l) after an infusion of 14–21 g of Hb, and the maximum concentration is reached 3–6 h after injection (Fairley 1940). A similar rise was noted in a subject injected with 16 g in whom the maximum plasma Hb concentration was 3.8 g/l (Gilligan et al. 1941). One gram of Hb is converted to 40 mg of bilirubin (With 1949). Therefore the catabolism of 16 g of Hb should yield 640 mg of bilirubin. If this were liberated into the plasma of an adult who was incapable of excreting bilirubin, the plasma bilirubin concentration would rise by about 10 mg/dl (170 μmol/l), assuming that about one-half of the liberated bilirubin diffused rapidly into the extravascular fluid space (Weech et al. 1941). In practice the bilirubin is delivered to the circulation over a period of several hours and excretion almost keeps pace with production.
Extravascular Destruction
Destruction by Antibodies That Are Slowly Lytic or Only Occasionally Lytic in Vitro
Lewis Antibodies
Many examples of anti-Lea and a few examples of anti-Leb lyse untreated red cells in vitro although lysis occurs only slowly. When small amounts of Le(a+) red cells are injected into the circulation of a patient with potent anti-Lea the cells are normally cleared by the mononuclear phagocyte system (MPS) with the liberation of only traces of Hb in the plasma. Haemoglobinuria has been observed after the transfusion of relatively large amounts of Le(a+) red cells, perhaps because the amount of Hb released is greater, or possibly because, owing to slow clearance, the cells have time to undergo lysis in the circulation before clearance by the ‘overloaded’ MPS can occur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree