Chapter 32 Michael Richards Department of Paediatric Haematology, Leeds Children’s Hospital, Leeds, UK Haemolysis describes the pathological process whereby red cells are destroyed resulting in a shortened lifespan. Most haemolytic processes result in extravascular red cell removal in the reticuloendothelial tissues (mainly liver and spleen). Clinical features include variable anaemia and jaundice, splenomegaly, occasional hepatomegaly and the formation of bile pigment gall stones. Haemolysis may also occur within the bone marrow tissue when unstable red cell precursors are destroyed prior to release into the circulation (e.g. thalassaemia major). Intravascular haemolysis is generally more severe and may require critical care support. Patients may develop back or loin pain, acute renal injury and, in severe cases, cardiovascular collapse. Important causes of intravascular haemolysis are: Both haemolysis within the intravascular compartment and in the reticuloendothelial tissues will result in a rise in the levels of unconjugated bilirubin and urobilinogen in the faeces and urine. Intravascular haemolysis results in the release of free haemoglobin into the plasma, which may occasionally be detected but is usually conjugated to haptoglobin, which then reduces the concentration of free haptoglobin. Haptoglobin concentrations may be increased during an acute-phase response, and production is reduced in infancy; therefore, reduced haptoglobin levels are not always a reliable marker of haemolysis. Rarely, haemoglobin is excreted through the kidney and can be detected in the urine as free haemoglobin and later as haemosiderin within the urine. The serum lactate dehydrogenase (LDH) is typically raised. Examination of the blood film may show abnormal red cell morphology, the nature of which depends on the particular process leading to haemolysis. The pathogenesis of haemolysis in the infant and child encompasses similar generic considerations as are encountered in adults (see Chapter 1). Inherited disorders and maternal/foetal interactions, however, play an increased role compared to pathology that is more relevant to adults. Assessment of the cause of haemolysis requires focus on the family history especially adverse outcomes in previous siblings and a focus on dysmorphic somatic physical features that may indicate a specific syndrome. The causes of haemolysis may be differentiated into pathology that is intrinsic or extrinsic to the red cell and whether it is a congenital or acquired disorder (Table 32.1). Table 32.1 Causes of haemolysis. Immune haemolysis occurs as a consequence of binding of immunoglobulin molecules to the red cell surface followed by interaction with monocytes and complement and removal of these erythrocytes. Alloimmune haemolysis may occur as a consequence of incompatibility of the red cell of the neonate with maternal red cells in haemolytic disease of the newborn (HDN), or haemolysis of incompatible red cells following transfusion. Autoimmune haemolysis occurs when the host immune system becomes sensitized to its own red cell antigens. Maternal sensitization to an incompatible foetal blood group antigen results in transfer of immunoglobulin IgG from the maternal to the foetal circulation, leading to haemolysis. Severely affected foetuses may have foetal hydrops. This is characterized by severe anaemia leading to foetal heart failure, progressively greater extramedullary erythropoiesis, hepatomegaly and splenomegaly. Hypoalbuminaemia, pericardial and pleural effusions and associated polyhydramnios may also occur. In mild disease, there may be no anaemia or anaemia well compensated for in utero. The resulting neonatal unconjugated hyperbilirubinaemia may lead to neurotoxicity, a condition known as kernicterus. The principle treatments for unconjugated hyperbilirubinaemia are phototherapy or exchange transfusions. Anaemia may be treated by top-up or exchange transfusions. The specific red cell antigens that may lead to severe haemorrhagic disease in the newborn include Rhesus D and c, Kell and Fya. Other antigen systems cause milder disorders; these include Rhesus E, C, Kpa, k and S. Anti-Kell antibodies may lead to the suppression of erythropoiesis. ABO group haemolysis is mild and has a negative or only weakly positive direct antiglobulin test (DAT) but prominent spherocytosis [1]. Treatment for HDN during the perinatal period depends on the severity of the condition. Significant hyperbilirubinaemia may develop within the first 24 h after birth, which may require phototherapy to avoid kernicterus. Severe anaemia and resistant hyperbilirubinaemia will require transfusion including possible exchange transfusion. AIHA can be divided into a primary state or secondary to a discrete underlying pathology. Primary AIHA is commonly due to warm reactive IgG autoantibodies, frequently with Rhesus antigen specificity that cause extravascular haemolysis predominantly in the spleen. The DAT is positive with anti-IgG. PCH follows the binding of the Donath–Landsteiner antibody, an IgG antibody that has specificity to the P red cell antigen. It binds in the peripheral circulation where temperatures are cooler (<30°C), but complement activation and intravascular haemolysis occur when the red cells pass to the warmer central circulation. The DAT results at room temperature are positive with anti-C3 (complement) but negative with anti-IgG. Cold agglutinin disease is characterized by the binding of IgM to the I or i red cell antigen at temperatures below 37°C and leads to avid complement fixation. Haemolysis may be either intravascular or extravascular. Spherocytes are most prominent in cases of warm autoantibodies. Red cell agglutination may be seen in cold AIHA. Reticulocytosis is common, but reticulocytopaenia may occur in approximately 10% of paediatric patients with AIHA. Very rarely a negative DAT can occur in a disorder that otherwise fits with an AIHA. Evan’s syndrome is characterized by AIHA, thrombocytopenia and rarely neutropenia, a severe disorder in which aggressive treatment is required. Warm AIHA and PCH frequently follow a viral-type illness. Cold AIHA is seen more commonly in adults and may follow mycoplasma infection. Most cases of AIHA have a good prognosis, and a proportion will remit spontaneously. In those cases with cold-reacting antibodies, maintenance of adequate body temperature including the peripheries is important. Treatment modalities for persistent AIHA include the use of red cell transfusions for significant anaemia. This is frequently technically challenging because the autoantibody may potentially mask a significant alloantibody during the crossmatching process. Treatment to alter the disease process includes immunosuppressives including corticosteroids and intravenous immunoglobulin and in IgM disorders exchange transfusion or plasmapheresis. Splenectomy has been used in chronic cases. There has been increasing evidence of the efficacy of rituximab, a therapeutic anti-CD20 antibody that leads to the lysis of B lymphocytes [2].
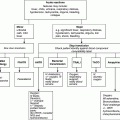
Haemolysis
Categorization of haemolytic processes
Congenital
Acquired
Intrinsic
to red cell
Membrane
abnormalities
Enzyme deficiencies
Haemoglobin
abnormalities
Thalassaemia
Haemoglobinopathies
Paroxysmal nocturnal
haemoglobinuria (PNH)
Extrinsic to
red cell
Immune
Autoimmune
Alloimmune
Microangiopathic haemolysis
Infections
Malaria
Babesiosis
Immune-mediated haemolytic anaemia
Haemolytic disease of the newborn
Autoimmune haemolytic anaemia (AIHA)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree