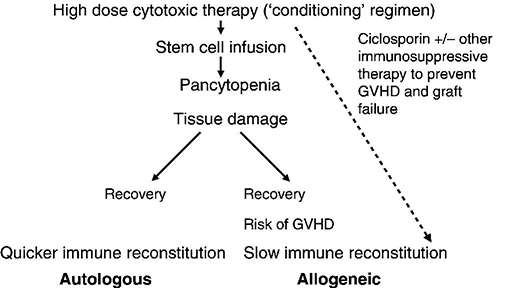
Chapter 21 John Snowden1,2 and Stephen Webber3 1 Department of Haematology, Sheffield Teaching Hospitals NHS Foundation Trust, South Yorkshire, UK 2 Department of Oncology, University of Sheffield, Sheffield, UK 3 Department of Anaesthesia and Critical Care, Sheffield Teaching Hospitals NHS Foundation Trust, South Yorkshire, UK Haematopoietic stem cell transplantation (HSCT) is a complex and toxic procedure [1], with a high risk of procedural mortality and serious morbidity reflected by 11–40% of patients subsequently requiring intensive care support [2]. The necessity for a close working relationship between the HSCT and critical care teams is highlighted by the fact that unhindered access to critical care support is now a mandatory accreditation requirement for HSCT programmes [3]. Intensive care specialists and their medical, nursing, pharmacy and allied health professional colleagues need to be familiar with the process of HSCT (Figure 21.1), the rationale for performing such procedures (Table 21.1) and the justification for exposing patients to a high risk of treatment-related complications. A working knowledge is not only essential for optimizing clinical outcomes in critically ill HSCT patients but also for effective communication with colleagues and families who are supporting the patient. Figure 21.1 Phases of haematopoietic stem cell transplantation (HSCT). HSCT is initiated by administration of the conditioning regimen, which consists of intensive cytotoxic chemotherapy +/− total body irradiation (TBI) and anti-lymphocyte antibodies (such as ATG or alemtuzumab). In autologous HSCT, the main aim is to dose intensify cytotoxic treatment to increase cancer cell killing and to use autologous cell infusion to hasten haematological recovery, which may not otherwise occur for months or years. Despite neutrophil recovery, patients may remain immunosuppressed due to the treatment or their underlying disease (myeloma, lymphoma). In allogeneic HSCT, the conditioning therapy has the dual purpose of destroying the underlying disease process and creating immunological space for the transplanted graft. Allogeneic HSCT also routinely requires the administration of additional immunosuppressant medication (such as ciclosporin) to prevent graft rejection and graft-versus-host disease (GVHD). GVHD may result in varying degrees of acute and chronic multi-organ dysfunction, which usually require immunosuppressive treatment. In addition, there is slow reconstitution of the transplanted immune system in the allogeneic HSCT recipient, potentially perturbed by GVHD and its treatments, and a long-term risk of infection susceptibility frequently persists. On the positive side, GVHD provides the additional dimension of an ongoing graft-versus-leukaemia/lymphoma (GVL) effect, which may contribute to long-term cure by elimination of low-level residual disease. This forms the principle of donor lymphocyte infusions (DLI), sometimes given post HSCT to maximize the GVL effect. Table 21.1 Indications for allogeneic and autologous HSCT in adults and paediatrics (with relative frequencies). Source: Passweg [4]. Copyright Nature. Reproduced with permission of Nature Publishing Group. The aim of this chapter is to familiarize the critical care reader with the process of HSCT and focus on specific complications that may lead to admission to critical care or otherwise feature in the clinical picture and require ongoing collaborative management. Inevitably, all types of HSCT render patients pancytopenic and at risk of neutropenic infection, septic shock, bleeding and a need for various transfused blood products. These aspects are considered in Chapters 20 (infection), 25 (shock), 3 and 15 (bleeding) and 13, 14 and 17 (transfusion). The practice of HSCT has grown massively since the first clinical bone marrow transplantation (BMT) procedures in the late 1960s. With the increasing use of other sources of haematopoietic stem cells (HSC), particularly peripheral blood stem cells (PBSC) and umbilical cord blood (UCB), the term HSCT is now more appropriate, although BMT persists in common parlance. Haematopoietic stem cell transplantation is an umbrella term referring to the reconstitution of the blood, bone marrow and immune systems by an infusion of HSC following the administration of intensive myelo- and/or lympho-ablative cytotoxic therapy (see Figure 21.1). HSCT may be from a donor, i.e. allogeneic HSCT, either within the family or unrelated, or from the patients themselves, termed autologous HSCT. Rarely, identical twins may be used (syngeneic HSCT). Various sources of HSC may be used, including the most traditional source of bone marrow harvested by direct aspiration under general anaesthetic, but now, the vast majority of HSC are PBSC mobilized with granulocyte colony-stimulating factor (+/− chemotherapy) and collected by apheresis, which have the advantage of quicker engraftment. The use of UCB, banked or as directed donations, is also on the increase, particularly in paediatric practice. The long-term risks of treatment-related mortality (TRM) and morbidity are substantially higher for allogeneic HSCT compared with autologous HSCT. Although individualized in practice, typical estimates of 1-year TRM given during the consent process are up to 3% for autologous HSCT but up to 20% for fully matched allogeneic HSCT and potentially higher for mismatched and UCB transplantation. Age and co-morbidities are also important considerations, although in recent years reduced intensity conditioning (RIC) regimens and better supportive care have extended the application of HSCT to older and less fit patients, including patients in their 70s. In every patient, the risks of TRM, serious morbidity and impact on quality of life have to be justified by clear potential for incremental survival over and above the alternative management options. This process should take place initially within the multidisciplinary team (MDT) meeting and then explained to the patient and their family during the consent process for HSCT. For example, provided the TRM risks are recognized, autologous HSCT can achieve cure in the majority of patients with aggressive non-Hodgkin’s lymphoma or Hodgkin’s disease in chemosensitive relapse, whereas the probability is much lower (at around 10%) with less intensive chemotherapy. By the same logic, the chances of long-term cure in many patients with poor-risk acute leukaemia are boosted by over 30% by allogeneic HSCT, although TRM risks are more substantial. Although the alternative treatment options may offer only remote chances of long-term disease control, this should not be a reason alone for offering HSCT. In addition, patients selected for autologous or allogeneic HSCT must be psychologically motivated and of sufficient physical fitness for an intensive and often complicated and protracted phase of treatment. Complications of HSCT may be categorized in a number of ways and depend on the type of HSCT. In both autologous and allogeneic HSCT, they include cytotoxic damage induced by the conditioning regimen to many tissues. The generation of pancytopenia leads to potential infective and bleeding complications and a dependency on transfused products. Other complications common to all intensive cytotoxic therapy, such as oropharyngeal and gastrointestinal (GI) mucositis, are frequently more pronounced in HSCT patients due to the higher intensity of cytotoxic regimen. Some complications are relatively unique or largely restricted to HSCT practice, particularly after allogeneic HSCT. Despite apparent haematological recovery, deficits in cell-mediated immunity typically persist for many months and potentially years following allogeneic HSCT, resulting in increased risk of acquisition or reactivation of a range of opportunistic viral and fungal infections. Allogeneic HSCT may also be associated with acute and chronic graft-versus-host disease (GVHD), a unique and broad spectrum of pathology requiring additional immunosuppressive treatment, which, in turn, adds to the state of infection susceptibility. Some HSCT patients therefore walk a fragile tightrope between infection and GVHD. Even after many years post transplant (and cure of their underlying disease), patients may destabilize with infection or other complications and require specialist critical care referral. While the mainstream complications of cytotoxic therapy are considered in Chapter 30, the following will cover those specific complications that directly result in or otherwise feature in the referral to critical care. Needless to say, there are frequently overlapping pathologies, and each patient is relatively unique, depending on their underlying condition, co-morbidities and degree of pre-HSCT treatment, type of transplant, donor source and compatibility and pre-existing parameters such as co-morbidities and viral status (Table 21.2). Table 21.2 Early and late complications of HSCT. Side effects of drugs may feature at any stage.
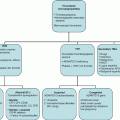
Haematopoietic Stem Cell Transplantation (HSCT)
Introduction
Allogeneic HSCT
Autologous HSCT
Acute myeloid leukaemia
31%
Myeloma and other plasma cell disorders
44%
Myelodysplastic syndrome/myeloproliferative diseases
16%
Non-Hodgkin’s lymphoma
31%
Acute lymphoblastic leukaemia
15%
Hodgkin’s disease
12%
Non-Hodgkin’s lymphoma
9%
Leukaemias
4%
Hodgkin’s disease
3%
Neuroblastoma
3%
Aplastic anaemia and other bone marrow failure syndromes
6%
Germinal tumours
2%
Myeloma and other plasma cell disorders
5%
Other solid tumours
3%
Chronic myeloid leukaemia
3%
Autoimmune diseases
1%
Chronic lymphocytic leukaemia
3%
Haemoglobinopathies (thalassaemia, sickle cell disease)
3%
Paediatric immunodeficiency diseases
3%
Others (including metabolic diseases, solid tumours, autoimmune diseases)
3%
Definitions and rationale for HSCT
Complications generating or featuring in a referral to critical care
Early (<3 months)
Late (>3 monthsn
Infection
Bacterial
Bacterial
Gram negative
Encapsulated bacteria
Gram positive (from central lines)
Viral
Viral
Reactivation (HSV, CMV, HHV-6, VZV, adenovirus, EBV)
Reactivation (HSV, VZV, EBV)
Acquired (RSV, influenza A and B, parainfluenza, norovirus)
Acquired (RSV, influenza A and B, parainfluenza, norovirus)
Fungal
Fungal
Aspergillus
Aspergillus
Candida
P. carinii ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access