Hormones
Anti-androgens (Prostate Cancer)
Gonadotrophin releasing hormone
Oestrogenic Agonists (diethylstilbestrol)
Anabolic steroids
Anti-ulcer therapy
Histamine 2 receptor blockers (cimetidine)
Proton pump inhibitors (omeprazole, esomeprazole)
Cardiac agents
Calcium channel blockers (verapamil, nifedipine, diltiazem, amlodipine)
Angiotensin Converting Enzyme inhibitors (captopril)
Cholesterol lowering agents (fenofibrate, simvastatin, fluvastatin)
Digoxin
Spironolactone
Neuroleptics
Wide variety of neurotransmitter agonists, antagonists and modulators
Tricyclic antdepressants
Diazepam
Phenothiazines
Antifungals
Ketoconazole
Cancer agents
Chemotheraputic agents
Alkylating agents
5 alpha reductase inhibitors
Antiretroviral therapy
Highly Active Antiretroviral Therapy (HAART)
Protease inhibitors
Nucleoside/non-nucleoside containing regimes
Recreational drugs
Alcohol
Cannabis
Heroin
Others
Methotrexate
Metronidazole
Metoclopramide
Phenytoin
Theophylline
Idiopathic/Pseudo/Obesity
Gynaecomastia developing in young adults and beyond is often multifactorial. Body fat increases relative to lean body mass as we age. Adipose tissue is an active site of extraglandular aromatization of testosterone to oestradiol and androstenedione to oestrone. In addition, there tends to be a gradual decrease in testosterone production by the ageing testes and an increase in sex hormone binding globulin, resulting in less free testosterone and a reciprocal increase in Luteinising hormone [22, 23]. These two factors probably account for most idiopathic gynaecomastia in the adult.
Obesity alone will also result in deposition of fat in the area of the breast. This Pseudogynaecomastia can be expected to reduce following weight loss. However, excessive weight loss following morbid obesity frequently leads to the development of a redundant, ptotic, skin fold that can only be corrected by surgery.
Familial
Differential Diagnosis
Male breast cancer, lipoma, neurofibroma, lymphangioma and vascular malformations (hamartomatous conditions), haematoma, dermoid cyst.
Pathogenesis
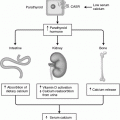
Gynaecomastia may be caused by a decrease in androgen production, an increase in oestrogen production, or increased availability of oestrogen precursors for peripheral conversion to oestrogen. The blockade of androgen receptors and increased binding of androgen to sex hormone binding globulin (SHBG) are other mechanisms [2]. Thus the circulating concentration of SHBG, the target cell response, and the extraglandular conversion of androgens to oestrogens are factors which might determine the development of abnormal breast tissue in the male.
SHBG is found normally in the circulation, binding the sex hormones tightly when compared with the weakly binding protein albumin. SHBG has a higher affinity for androgens than oestrogens; therefore oestrogens will be displaced more easily if an excess of hormone is present [26, 27].
Extraglandular conversion of androgens (testosterone and androstenedione) to oestrogen occurs in the liver, skin, fat, muscle, bone, and kidney. All these tissues contain an aromatase enzyme, responsible for conversion. The 17-hydroxysteroid dehydrogenase enzyme has a similar interconversion effect on androgens and oestrogens in extra glandular tissues [28, 29].
Testes and ovaries each secrete both oestrogen and androgen (testosterone). The production of these steroids or their precursors also occurs in the adrenal glands. An imbalance in the normal ratio of oestrogen to androgen causes an increase in glandular breast tissue [30].
There is no difference between the nature of male and female breast tissue hormonal response, which is individually led. By this, it is meant that breast proliferation depends on a particular individual’s hormonal constitution, and the duration, sensitivity, and intensity of hormonal stimulation.
Oestrogen causes breast ductal epithelial hyperplasia, ductal elongation and branching, proliferation of periductal fibroblasts, and an increase in vascularity. Separately, in females, progesterone causes acinar development, not seen in males [28].
The transient neonatal appearance of gynaecomastia relates directly to elevated oestrogen levels in the foetal circulation. The placenta transforms dehydroepiandrosterone and dehydroepiandrosterone sulphate to oestrone and oestradiol, stimulating breast glandular proliferation.
Histopathology
Glandular proliferation in the breast depends upon the intensity and duration of stimulation and has the same histological appearance regardless of aetiology.
Firstly, there is extensive ductal epithelial hyperplasia, proliferation and lengthening of the ducts, an increase in the stromal and periductal connective tissue, and proliferation of periductal inflammatory cells and fibroblasts. This early oedematous stage lasts for approximately 6 months and the breast can be painful or tender to palpation [19].
With time (12 or more months) the inflammatory reaction subsides and discomfort usually settles. There remains an increase in the more dilated ducts with a commensurate increase in stroma that may show frank histological fibrosis at this later stage [31, 32].
This dense fibrous glandular tissue forms directly beneath the nipple–areolar complex, and is supported by a fibrofatty stroma peripherally [33]. This stroma may develop further as the BMI of the patient increases. Medical management is much more effective in the early stages before fibrosis has developed.
Diagnosis
All patients presenting with proliferation of tissue in the breast area require a detailed history and examination. Often this is all that is necessary to obtain an accurate diagnosis.
The history should include duration of symptoms, presence of discharge, pain, or skin changes. The age of the patient, the pubertal development, and presence of testicular masses should also be queried. The past medical history and general health is of great importance and may reveal evidence of liver, kidney or thyroid disease, and current medication including possible non-prescribed drugs are of considerable relevance.
A careful and sympathetic psychosocial history should also be taken. The psychological sequelae of male breast enlargement may be the main reason for presentation in children over the age of around 7–8 years, when ‘visible difference’ from peer groups becomes a source of possible distress [9].
Examination is general and specific. BMI calculation and the search for signs of liver, kidney, and thyroid disease is mandatory. Examination of the genitals for abnormalities and development and palpation the regional lymph nodes is also indicated.
The breast is observed for size, shape, symmetry, and skin appearance. The patient is asked to lie supine and place their hands behind their head. The breasts are then examined with thumb and forefinger, gradually moving the digits towards the nipple [1]. In true gynaecomastia a rubbery or firm, mobile, disc of tissue will be identified concentrically beneath the nipple–areolar complex. This mass can normally be detected when greater than 0.5 cm in diameter [34]. If no such mass is palpated in the enlarged male breast then a diagnosis of pseudogynaecomastia is confirmed. The presence of an eccentric, fixed, hard, or unilateral growth requires differential diagnostic consideration.
Laboratory testing, radiological studies and FNA/Core biopsy are not required on adolescents when idiopathic gynaecomastia is diagnosed. Adult males presenting without an obvious cause commonly undergo investigation. Radiological imaging in these less frequent cases should include ultrasound scan or mammogram and a FNA or core biopsy to complete the ‘triple assessment’, similar to the female with a breast lump.
Laboratory tests include liver, kidney and thyroid function tests. Hormonal testing measures levels of testosterone, oestradiol, prolactin, luteinising hormone, and hCG. Results can direct towards pituitary, gonadal, and extragonadal causes [6].
Further investigations in the search for a more sinister causative diagnosis can include testicular ultrasound scan, abdominal CT scan (imaging adrenal glands) or an MRI scan of the brain (imaging the sella turcica) [34].
Classification
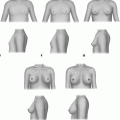
There have been numerous classification systems; therefore we have included this in Table 29.2 [35]. In practice, clinical judgements are based on individual factors that include psychosocial aspects.
Table 29.2
Gynaecomastia classification systems
Authors | Classification |
|---|---|
Nydick 1961 | Gland limited to retroareolar region |
Gland extends to edge of areola | |
Gland extends beyond areola | |
Tanner 1971 | 1. Nipple prominence |
2. Mammillary button stage; breast and areola slightly swollen | |
3. Further swelling breast and areola | |
4. Protrusive areola and nipple above the breast | |
5. Protrusion of nipple only after retraction of the areola | |
Simon 1973 | 1. Small visible breast enlargement; no skin redundancy |
2a. Moderate breast enlargement without skin redundancy | |
2b. Moderate breast enlargement with skin redundancy | |
3. Marked breast enlargement and skin redundancy (pendulous) | |
Deutinger 1986 | 1. Thoracic wall poor in flesh; mammary tissue localised behind nipple; no skin excess |
2. Adipose thoracic wall; widespread alterations, feminine breasts | |
3. Widespread alterations; excess adipose tissue, skin redundancy inframammary fold, ptosis | |
Cohen 1987 | 1. Glandular gynaecomastia |
2. Glandular gynaecomastia with ptosis | |
3. Adipose gynaecomastia | |
4. Adipose gynaecomastia with slight glandular component | |
Rohrich 2003 | 1. Minimal hypertrophy without ptosis |
2. Moderate hypertrophy without ptosis | |
3. Severe hypertrophy with ptosis grade 1 | |
4. Severe hypertrophy with ptosis grade 2 or 3 | |
Cordova 2004 | 1. Increase in diameter and protrusion limited to areolar region |
2. Areola–nipple complex above inframammary fold | |
3. Areola–nipple complex at level of inframammary fold | |
4. Areola–nipple complex below inframammary fold |
Treatment
Neonatal
Most babies with neonatal gynaecomastia will be identified in the immediate post natal examination, and the family reassured. Immediate investigation in this age group is usually not indicated unless the condition is accompanied by other abnormal findings suggestive of a congenital anomaly or syndrome.
First Decade
Children presenting with gynaecomastia in the first decade of life will usually have either obesity related pseudogynaecomastia, or a more specific cause (often pharmacologically induced) which will be evident from knowledge of the child’s other conditions. Presentation at this age is most frequently to a General Practitioner or Paediatrician, and requires a full appraisal as described, with referral to a specialist paediatric endocrinologist in most cases if the BMI is normal. Referral to a paediatric general or plastic surgeon for an early opinion may be indicated at this time. Even if surgery is deferred until the adolescent period, careful consideration should be given to the merits of surgery to alleviate serious teasing and bullying around the common school transfer age of 11. Surgery in the first decade is uncommon, but in severe cases may be the only means to address a highly visible external deformity and be the most valuable option for management. Surgical management is described below.
Endocrine management of children in whom gynaecomastia has been the presenting sign of a more general condition is beyond the scope of this chapter. However, the need for long-term definitive management of hormonal or other disorders should not preclude the use of surgical intervention where it might prove to be the single most effective intervention in improving the child’s overall health and well being.
Second Decade
Adolescents presenting with gynaecomastia frequently seek help from diverse routes. Although initial advice from a GP might be sought, many such affected young people are already deeply concerned about the developing condition and avoid contact with familiar and more conventional sources of help. Direct presentation to a plastic surgeon is common, and the proliferation of easily accessible sources of information from the internet and mass media has served to facilitate this diversification of possible sources of help. This is something of a mixed blessing; information streams are of variable quality and accuracy, and the commercial economy of much global health provision offers potential for the exploitation of vulnerable and inadequately advised young people. Many affected teenage boys become greatly distressed by what they perceive as dismissive and unsympathetic first consultations if they are merely advised that the condition will disappear and they should simply cope with their condition without skilled counselling or psychological advice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree