Stage I: tumor confined to ovaries
IA
Tumor limited to 1 ovary, capsule intact, no tumor on surface, negative washings
IB
Tumor involves both ovaries otherwise like IA
IC
IC1: surgical spill
IC2: capsule rupture before surgery or tumor on ovarian surface
IC3: malignant cells in ascites or peritoneal washings
Stage II: tumor involves 1 or both ovaries with pelvic extension (below the pelvic brim) or primary peritoneal cancer
IIA
Extension and/or implant on the uterus and/or fallopian tubes
IIB
Extension to other pelvic intraperitoneal tissues
Stage III: tumor involves 1 or both ovaries with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes
IIIA
Positive retroperitoneal lymph nodes and/or microscopic metastasis beyond the pelvis
IIIA1
Positive retroperitoneal lymph nodes only
IIIA1(i)
Metastasis ≤10 mm
IIIA1(ii)
Metastasis >10 mm
IIIA2
Microscopic, extrapelvic (above the brim) peritoneal involvement ± positive retroperitoneal lymph nodes
IIIB
Macroscopic, extrapelvic, peritoneal metastasis ≤2 cm ± positive retroperitoneal lymph nodes. Includes extension to capsule of the liver/spleen
IIIC
Macroscopic, extrapelvic, peritoneal metastasis >2 cm ± positive retroperitoneal lymph nodes. Includes extension to capsule of the liver/spleen
Stage IV: distant metastasis excluding peritoneal metastasis
IVA
Pleural effusion with positive cytology
IVB
Hepatic and/or splenic parenchymal metastasis, metastasis to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity)
Table 17.2
FIGO endometrial cancer staging 2014
IA | Tumor confined to the uterus, no or <½ myometrial invasion |
IB | Tumor confined to the uterus, >½ myometrial invasion |
II | Cervical stromal invasion, but not beyond uterus |
IIIA | Tumor invades the serosa or adnexa |
IIIB | Vaginal and/or parametrial involvement |
IIIC1 | Pelvic node involvement |
IIIC2 | Para-aortic involvement |
IVA | Tumor invasion bladder and/or bowel mucosa |
IVB | Distant metastases including abdominal metastases and/or inguinal lymph nodes |
Morphological Features
Uterine carcinosarcoma is typically a polypoid, bulky mass filling the entire uterine cavity, with a hemorrhagic and necrotic component. Myometrial invasion is frequent as well as extension beyond the uterus. Ovarian carcinosarcoma is also typically a very large tumor with massive areas of hemorrhage and necrosis. The morphological features and biology of this tumor appear identical irrespective of its site of origin in the female genital tract [3].
Histologically (Fig. 17.1), the tumor is biphasic, with both malignant epithelial and mesenchymal elements. The carcinomatous component comprises an admixture of high-grade carcinomas of endometrioid grade 3, serous, clear cell, or undifferentiated features. The sarcomatous component is either homologous or heterologous. Homologous sarcoma comprises high-grade undifferentiated round cell or spindle cell sarcomatous proliferation, with some features similar to an endometrial stromal sarcoma or fibrosarcoma. Heterologous elements, which are seen in approximately 50 % of cases, may show cartilaginous, osteosarcomatous, rhabdomyosarcomatous, or liposarcomatous differentiation. Neural or angiomatoid differentiation may also be seen. Myxoid change with hyaline globules is a prominent feature. The proportion of each carcinoma or sarcoma component may vary from one tumor to another [3].
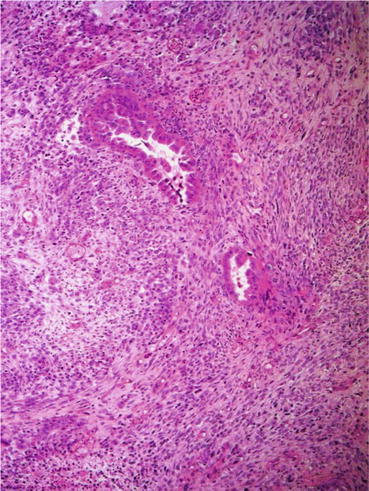

Fig. 17.1
Uterine carcinosarcoma
The histology of the metastatic component is more in keeping with an epithelial origin, since myometrial and lymphovascular invasion often display an epithelial morphology. The metastatic tumors show an epithelial component, in approximately 70 % of cases, while both carcinomatous and sarcomatous elements are found in 25 % of cases and sarcoma in only 6 % of metastatic tumors [4]
Molecular Genetics
The histogenesis of female genital tract carcinosarcomas has been debated and several theories have been proposed, including the collision between a carcinoma and an adenosarcoma, and the combination theory, in which both components arise from a single stem cell clone. However, the conversion theory postulating that sarcoma derives from carcinoma is currently favored [4]. Recent immunohistochemical and molecular findings support the hypothesis that gynecological carcinosarcomas are metaplastic carcinomas. Cell lines established from carcinosarcomas are able to differentiate into either epithelial components, mesenchymal components, or both [5]. Immunohistochemistry demonstrates the expression of epithelial markers in the sarcomatous component of carcinosarcoma. Moreover, clonality study patterns, genomic analysis, and loss of heterozygosity (LOH) studies have shown that the carcinomatous and sarcomatous components of these tumors share common genetic alterations and are monoclonal [6]. The transformation of a carcinoma to a sarcoma in these tumors may represent transdifferentiation as seen in epithelial to mesenchymal transition phenomena [7]. Several studies have demonstrated the expression of EMT-related genes in these tumors. A loss of epithelial characteristics, including an E-cadherin to N-cadherin switch, and an acquisition of mesenchymal phenotype were seen along with changes in the miRNA expression profile and the upregulation of all the E-cadherin repressors analyzed. Specifically, the miR-200 family appears to be a key regulator of EMT, through the downregulation of the E-cadherin repressors Zeb1 and Zeb2, thereby maintaining the epithelial phenotype. In addition, phospho-Akt (which plays a key role in EMT) is increased in the mesenchymal component and correlated negatively with E-cadherin expression, and this was associated with significant upregulation of an EMT transcription factor, Slug [8].
The molecular alterations seen in uterine carcinosarcomas are more akin to type II non-endometrioid than type I endometrioid uterine carcinomas. Data concerning molecular alterations in ovarian and uterine carcinosarcomas are scarce and based on analysis of a relatively small number of samples [9]. TP53 mutations and/or protein overexpression is considered to be the most frequent events with p53 positivity observed in up to 60 % of tumors and TP53 mutations in 23 % of cases [10]. PI3KCA mutations have also been reported in 19 % of uterine carcinosarcomas and KRAS mutations in 24 % [11]. Contradictory results have been found with PTEN mutations: 0–14 %. In rare cases, mutations affecting β-catenin (7 %) and NRAS (2 %) have been identified. Studies have demonstrated that up to 45 % of uterine carcinosarcomas express Abl, 19 % HER-2/neu, 100 % PDGF-R β, 32 % ER-β, and 23 % EP-B. Overexpression of Cox-2 (33 %), EGFR (30 %), Trop-2 (35–57 %), c-KIT (16–25 %), TGF-β, and PARP has also been reported. VEGF is strongly expressed in uterine carcinosarcomas [12]. Consistent with the high frequency of P53 alterations, most uterine carcinosarcomas exhibit high chromosomal instability. Cytogenetic studies of uterine carcinosarcomas have revealed extremely complex karyotypes with gross chromosomal anomalies, such as polysomy 8. Comparative genomic hybridization studies have demonstrated gains and losses at multiple chromosomal loci [6]. Gains (85 %) were observed more frequently than losses (30 %). The most frequently occurring CGH changes were gains on chromosomes 1q, 2p, 8q, 12p, 19q, and 20q and losses on 4q, 9q, and 13q.
Prognosis
Female genital tract carcinosarcomas have a very poor prognosis with overall 5-year survival of less than 30 %.
Uterine Carcinosarcoma
Although stage I uterine carcinosarcoma has a better prognosis (50 % of 5-year overall survival), it is still significantly worse than in stage I high-grade endometrial carcinoma (80 % 5-year overall survival) [1]. Median overall survival varies from 8 to 26 months [13]. Most patients experience a relapse within 1 year after completion of treatment. The FIGO stage, patient’s age (over 55), depth of myometrial invasion, and patient’s race are the most frequently reported prognostic factors in uterine carcinosarcoma. Lymph node dissection, tumor size, lymphovascular space invasion, parity, and grade of the sarcomatous compound have a less certain prognostic value, while data on the presence of heterologous elements or previous pelvic radiotherapy are contradictory [12–15].
Ovarian Carcinosarcoma
Overall, the prognosis for ovarian carcinosarcomas appears worse than in uterine carcinosarcomas [13] even if controversial [14] and surely worse than in high-grade ovarian carcinomas of a similar FIGO stage [16]. Most (90 %) present with an advanced disease (>stage I), and the median overall survival ranges from 7 to 27 months. Five-year overall survival is only 7–20%for patients with advanced-stage (III or IV) disease.
For ovarian carcinosarcomas, the FIGO stage is the strongest prognostic factor. Some reports indicate that complete cytoreduction, advanced age, sarcomatous component grade, and the use of adjuvant chemotherapy are prognostic factors [16]. It should be noted that the limited number of patients, with various regimens used over an extended period, and the lack of central pathological review in such retrospective studies make it impossible to draw definitive conclusions.
Initial Treatment
Optimal treatment remains uncertain. Ovarian and uterine carcinosarcomas are routinely excluded from upfront clinical trials. Treatment recommendations are mainly based upon retrospective studies with small patient populations especially for ovarian carcinosarcomas.
Surgery
Uterine Carcinosarcoma
Primary treatment includes peritoneal lavage for cytology, total abdominal hysterectomy, bilateral salpingo-oophorectomy with dissection of the pelvic and para-aortic lymph nodes, and maximal tumor debulking. Surgical staging for these tumors should follow the procedures performed for ovarian carcinoma, including detailed examination of the entire abdominal cavity and retroperitoneal spaces and appropriate biopsies. Although the role of omentectomy is unclear, it is recommended in women with early-stage disease. The role of lymphadenectomy is a subject of current debate. However, given the relatively high incidence of lymph node involvement (14–38 % in early stage), regarding impact on survival, the majority of the retrospective studies suggest a significant survival benefit of the lymph node dissection in uterine carcinosarcomas [17, 18]. Nemani et al. [17] reported a significant OS benefit associated with lymph node dissection, with a 5-year OS of 49 % versus 35 % for patients who had not undergone lymph node dissection. Therefore, adequate lymphadenectomy appears necessary for both staging and therapeutic reasons. In advanced disease, primary cytoreductive surgery is generally performed, despite the lack of clear evidence. In a recent series, cytoreductive surgery was found to improve overall survival in patients with advanced carcinosarcomas [19].
Ovarian Carcinosarcoma
Cumulative retrospective data support the benefit of an optimal surgical cytoreduction with total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, abdominal fluid aspiration, pelvic and para-aortic lymph node dissection, and tumor debulking. Given the rarity of ovarian carcinosarcomas, the role of cytoreductive surgery has not been prospectively evaluated. Several small retrospective studies with fewer than 50 patients have reported an improved outcome for patients undergoing an optimal debulking surgery, without residual disease. One of the largest studies, including 50 patients, reported DFS of 19 months for patients with only microscopic disease, compared to 10 months for those with less than 1 cm residual disease and 5 months for those with more than 1 cm (p = 0.01). Overall survival is 47, 18, and 8 months, respectively (p = 0.02) [16]. From the SEER database, Garg reported improved survival for patients with lymphadenectomy suggesting the benefit of lymph node dissection, although this may reflect stage migration. The risk of death was reduced by 34 % after lymphadenectomy (HR = 0.66, 95 % CI = 0.56–0.78) [13]. Conservative surgery is never indicated for ovarian carcinosarcoma even in adequately staged stage IA disease.
Adjuvant Treatment for Early Stage
Due to the high rate of local and distant recurrences, even for early-stage disease, adjuvant systemic treatment is generally considered. There is still no clear consensus on the best adjuvant therapy for patients with carcinosarcoma as most studies are retrospective and describe the outcome in a small number of patients who were given a variety of treatment regimens.
Uterine Carcinosarcomas
Adjuvant Radiotherapy
Pelvic recurrence is common, even for patients with early-stage disease; therefore, pelvic radiotherapy (with or without brachytherapy) has been commonly used and reduces the incidence of local pelvic recurrence [20]. However, its impact on patient survival is not proven and remains controversial. The only phase III study comparing pelvic radiotherapy and observation is Reed’s EORTC study.
Two hundred and twenty-four (224) FIGO stage I–II uterine sarcomas, including 91 carcinosarcomas, were randomized between observation and RT. Analysis of all patients revealed a reduction in local relapse (p = 0.004) but no effect on either overall or disease-free survival. The local recurrences rate was 18.8 % for patients treated with radiotherapy and 35.9 % in the observation arm. The same results were observed among patients with carcinosarcomas. However, most patients relapsed simultaneously at distant sites, and therefore radiotherapy appears to be of limited value [20].
The SEER database from Wright recorded 1819 patients with stage I–II uterine carcinosarcomas and reported, in a multivariate model, a 21 % reduction of death for women who underwent radiotherapy. The benefit was only observed for women who did not undergo lymph node dissection [21]. In the second study using also SEER data (n = 2461), Clayton Smith reported an improvement in overall survival for women with uterine carcinosarcomas treated with postoperative radiotherapy compared to surveillance. The overall 5-year survival was 41.5 %, using adjuvant radiotherapy compared to 33.2 % (p < 0,001) [22]. However, a third SEER analysis (n = 1855) did not show any impact of radiotherapy on further prognosis (also in the group of patients without lymphadenectomy) [17]. Large database reviews present limitations because of the lack of standardization in surgery, radiotherapy, and chemotherapy, the absence of centralized pathological review, and the potential impact of patients and physicians preference on adjuvant treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree