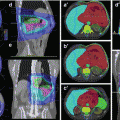
Fig. 15.1
Lymphatic drainage of the cervix. The lymphatic trunks are presented and lymph node echelons are tabulated. 2Obturator ln are part of the medial subgroup of the external ilian ln, located close to the obturator internus muscle. 1The term hypogastric node is used variably, to describe the most cephalic of the internal iliac nodes or the entire internal iliac node chain (2). Adapted from Plentl AA, Friedman EA, Lymphatic system of the female genitalia. Philadelphia. WB Saunders, 1971 p.83.
15.1.3 Imaging Modalities
15.1.3.1 Computed Tomography
CT remains the most widely used diagnostic imaging modality for staging and is the most frequently employed imaging modality for target delineation in cervical cancer. It is excellent in delineating lymph node regions (although its accuracy to differentiate normal from tumor-involved nodes is inferior to PET/CT). CT readily depicts the morphology of presacral target tissues and normal structures, including the small and large bowel, bladder, other abdominal organs, kidneys, and bone. CT is superior in delineating surgical clips and implanted vaginal gold markers. CT allows delineation of the uterus but does not differentiate tumor from the normal uterus, which generally is sufficient for the purposes of external beam radiation. However, substructures of the uterus, cervix, isthmus, corpus, and fundus are challenging to delineate on CT. Because of its lacking tissue differentiation between the tumor and normal soft tissue structures, CT is limited in delineating the tumor extent within the uterus. Its accuracy in parametrial involvement is inferior compared to MRI (Mitchell et al. 2006; Subak et al. 1995). Therefore, MRI is the preferred imaging modality for high-precision delineation of the primary tumor/extent as required for image-guided brachytherapy.
15.1.3.2 Magnetic Resonance Imaging (MRI)
High-precision assessment of the primary tumor volume and extent is best assessed by MRI in cervical cancer (Bhosale et al. 2010; Balleyguier et al. 2011; Hricak 1991). With its superior soft tissue contrast, MRI is superior to CT in delineating the tumor within the cervix and differentiating it from the uterus and adjacent pelvic tissues, including bladder invasion, parametrial extension, and rectal and perirectal involvement (Mitchell et al. 2006; Subak et al. 1995). This high precision is particularly important for image-based brachytherapy, more so than in external beam radiation, where target volumes include the parauterine structures. Vaginal extension is more accurately delineated by MRI (Sala et al. 2007). MRI is superior to CT in assessing serial subtle morphologic changes in tumor volume/extent (Mayr et al. 2010), which is relevant for longitudinal imaging during therapy for adaptive therapy and for image-guided brachytherapy.
For the assessment and morphologic depiction of regional lymph nodes and postoperative seromas, CT and MRI are generally equivalent (Mitchell et al. 2006; Subak et al. 1995). For both CT and MRI, the identification of involved lymph nodes is limited to morphologic criteria, including shape and internal architecture. Configuration criteria for involvement include size (>1 cm in short axis), short-axis-to-long-axis ratio (i.e., more round shape is more consistent with involvement), and margin irregularity. Architectural criteria include central necrosis on contrast-enhanced CT (Yang et al. 2000) or MRI, signal intensity irregularity on T2-weighted MRI (Brown et al. 2003), and loss of the fatty hilum (Koh et al. 2006). With the overall limited accuracy, a combination of size, configuration, and internal architecture should be used.
MRI is helpful in identifying incidental, non-tumor-related findings that may influence therapy including distortion or retroversion of the uterus, fibroids, ovarian findings, and other pelvic abnormalities. The cervical tumor is best delineated on T2-weighted imaging, fast spin echo, or turbo spin-echo sequences, which provide high resolution, while gradient echo sequences are more susceptible to artifacts. T2-weighted sequences are superior to T1-weighted and contrast-enhanced imaging (Sironi et al. 1993). Diffusion imaging may be helpful, particularly when T2-weighted images are equivocal (Charles-Edwards et al. 2008). The use of 3 T MRI does not improve the accuracy over 1.5 T (Hori et al. 2009).
15.1.3.3 Positron Emission Tomography (PET)
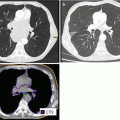
The staging and target delineation of cervical cancer have been further advanced by the introduction of fluorodeoxyglucose (18F)(FDG) PET and PET/CT. While CT and MRI identify lymph node involvement, assessment is limited to morphologic size and architectural criteria. The metabolic characterization of lymph nodes by PET/CT provides the most sensitive assessment of pelvic and para-aortic lymph node involvement.
15.1.3.4 Imaging–Surgical–Pathology Correlations
In imaging–surgical–pathology correlation studies, CT has been reported to have an accuracy of 70–80 % for evaluation of lymph node involvement based on size. Accuracy improves to 93 % with a cutoff value of 1 cm (Hawnaur 1993; Hardesty et al. 2001; Bellomi et al. 2005).
In MRI–pathology correlation studies, MRI has been shown to provide better staging than clinical exam in early stage cervical cancer (Bhosale et al. 2010; Balleyguier et al. 2011; Hricak 1991). For tumor volume and delineation, MRI–histologic correlation was found to be 98 % in comparisons of tumor volume from 3D MRI with digitized giant histologic tissue sections (Burghardt et al. 1989; Greco et al. 1989). These studies established the high accuracy of MRI for the delineation of tumor extent in cervical cancer. In surgical–pathology correlation studies, MRI showed better sensitivities (40–57 %) and specificities (77–80 %) than CT for parametrial invasion (Siegel et al. 2012).
The sensitivity for PET ranges from 79 to 91 % (Siegel et al. 2012) with most misses related to microscopic involvement (Lin et al. 2003). PET/CT, with the added spatial resolution from CT, has a sensitivity of 58–72 %, a specificity of 93–99 %, and an accuracy of 85–99 % for lymph node involvement in cervical cancer (Choi et al. 2006; Sironi et al. 2006).
15.1.4 Target Delineation
15.1.4.1 Simulation Procedure
For cervical cancer, it is critical to incorporate the information from both imaging and clinical (pelvic) examination into the radiation therapy planning. Visible and/or palpable and mucosal vaginal involvement is essentially impossible to assess by axially oriented CT imaging and can also be missed on MRI. Placement of radiopaque seed markers into the cervix or into the most distal visible or palpable vaginal tumor extent assists the delineation and the target volume design. Gold markers are recommended for this purpose as ferromagnetic materials will cause metallic artifacts on MRI preventing proper imaging. A dry tampon, which provides negative contrast for CT or MRI, is a simple method to delineate the vagina and lower extent of the cervix/tumor. However, rigid vaginal markers, rectal markers, and rectal contrast administration are not recommended as they may cause upward displacement of the cervix and distension of the rectum with ensuing displacement of the cervix, respectively. If target delineation is based on such displaced anatomy, geographical tumor miss may occur during actual treatment, as daily therapy is delivered without markers/rectal distension. Vaginal tampons have been shown to not cause displacement (Weidner et al. 1999). If excessive rectal distention is noted at the time of simulation, repeat scanning after evacuation or bowel preparation regimen may increase target reproducibility. This is particularly important in postoperative radiation therapy, where mobility of the vaginal target has been well documented and overall target volumes in the pelvis tend to be smaller (Chan et al. 2008; Taylor and Powell 2008).
For CT simulation, intravenous contrast is helpful for the differentiation of regional lymph nodes from vessels, depiction of nodal architecture (see Sect. 1.3.2), and delineation of the bladder. Oral contrast given 1–1.5 h before the simulation can improve differentiation of the bowel from lymph nodes (Figs. 15.2, 15.3, 15.4, 15.5, 15.6, and 15.7), vascular structures, and tumor. However, the effect on dose calculations (Williams et al. 2002) has to be addressed during dosimetry by correcting the inhomogeneity value of the contrast-opacified bowel to water density. For MRI simulation, T2-weighted sequences to delineate the primary tumor, parametria, vagina, and lymph nodes without contrast are used, as the bladder and bowel are readily delineated on these sequences. Contrast is not needed for primary tumor delineation.
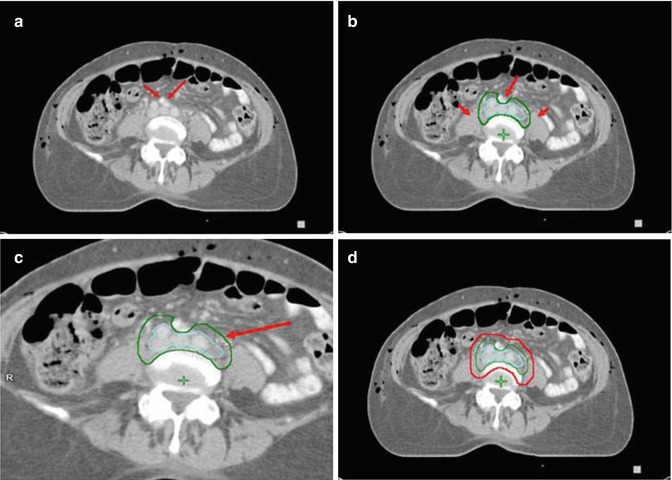
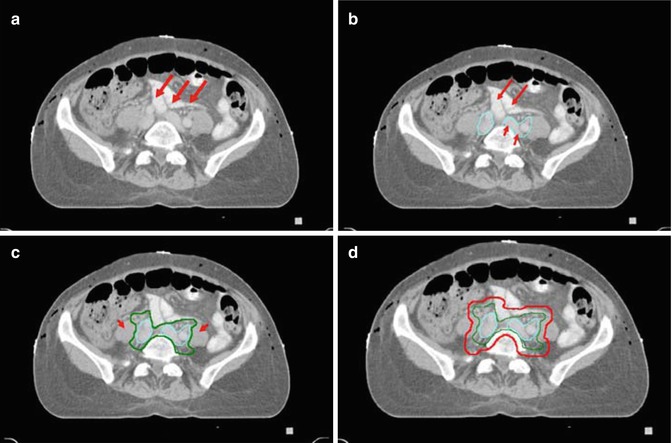
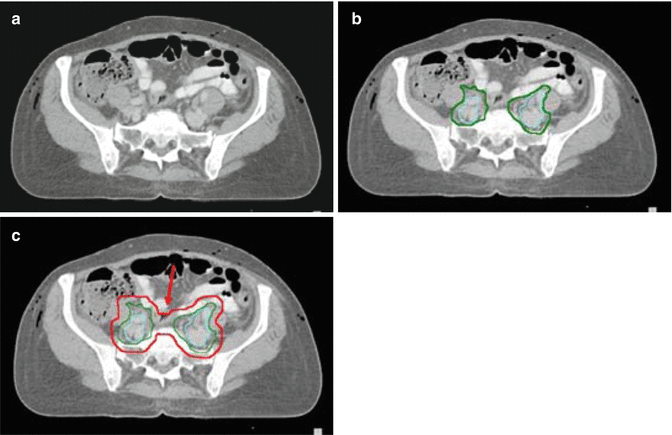
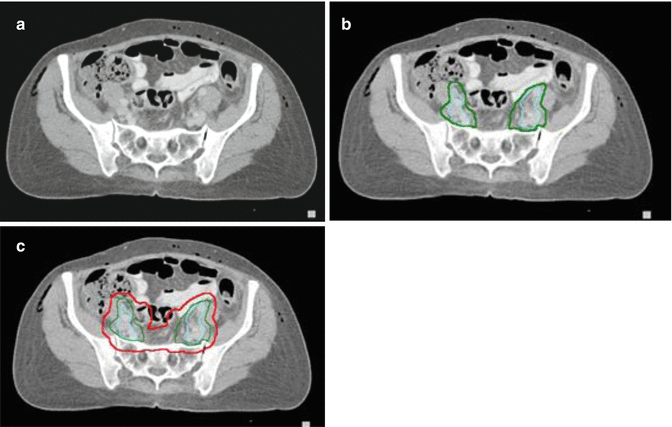
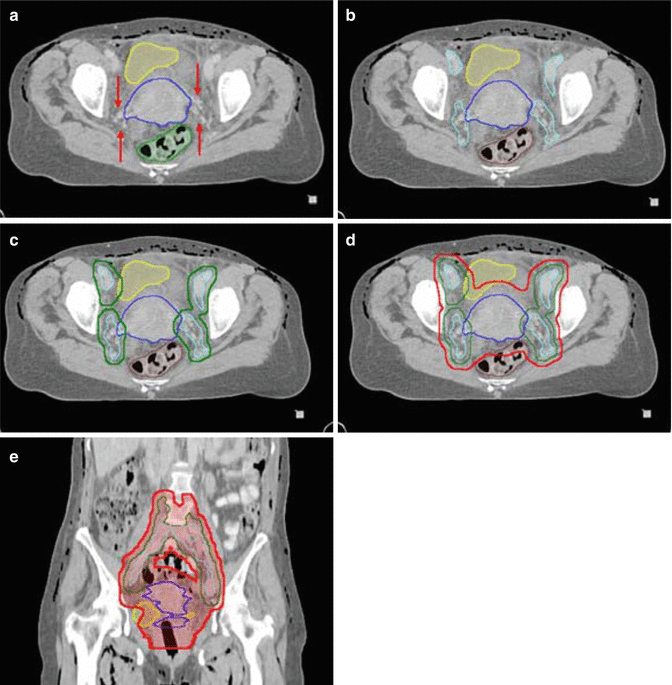
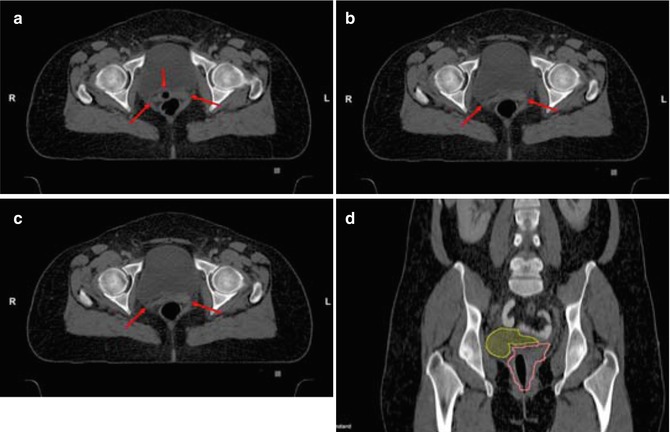

Fig. 15.2
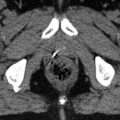
Contouring of the upper common iliac lymph node target. (a) The right and left common iliac arteries and veins are seen at the level just below the aortic bifurcation. The different contrast properties of the barium in the small bowel, compared to vascular contrast, help differentiate bowel from vessels and nodal structures. (b) The vessels and adjacent lymphatic regions have been contoured (aqua) for reference. The lymph node CTV is shown in green. (c) Separate delineation of the vessels and lymph nodes (aqua) may not be needed. Instead the lymph node CTV (green) can be outlined directly at a distance of 0.7 cm from vessels/nodes using the circle cursor (arrow), set at 0.7 cm, or a similar drawing tool (c), simultaneously excluding psoas, bowel and bone in uninvolved lymph node sites. (d) For the lymph node PTV (red) a margin of 0.7–1 cm is added to the CTV

Fig. 15.3
Contouring of the mid common iliac lymph node target. (a) The right and left common iliac arteries, veins and lymph nodes are seen medial to the psoas muscles with bowel (arrows) in close proximity. (b) The left common iliac vein (short arrows) is sectioned longitudinally. Vessels and adjacent lymphatic regions have been contoured (aqua) for reference. (c) The CTV (green) again adds a 0.7 cm margin to vessels and adjacent lymphatic tissue, excluding small bowel and psoas. (d) For the lymph node PTV (red) an additional margin of 0.7 cm is added to the CTV

Fig. 15.4
Contouring of the upper external and internal iliac lymph node target. (a) At the level just below the bifurcation of the common iliac arteries and veins, the right and left external and internal iliac arteries, veins and lymph nodes are seen medial to the psoas muscles. (b) Vessels and lymph nodes are contoured for reference (aqua). (c) The CTV (green) adds a 0.7 cm margin to vessels and adjacent lymphatic tissue, excluding small bowel and psoas. (d) For the lymph node PTV (red) adds an additional margin of 0.7. In addition a 2 cm strip of tissue anterior to the sacrum (arrow) is added to include the presacral lymph nodes

Fig. 15.5
Contouring of the mid external and internal iliac lymph node target. (a) Below the level in Fig. 4 external iliac vessles and nodes are seen anteriorly, internal iliac posteriorly and con. (b) Vessels and lymph nodes and lymph node CTV (green) are contoured as in Figs. 3–4. (d) For the lymph node PTV (red) adds an additional margin of 0.7. and a 2 cm strip of tissue anterior to the sacrum as in Fig. 4

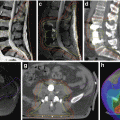
Fig. 15.6
Contouring of the GTV and distal lymph node target. (a) The cervical tumor and uterus (blue), bladder (yellow) and rectum (light green) are contoured. Precise delineation of paramtrial extension is challenging with CT and indistinguishable fro parametrial lymph nodes and vessels. (b) Delineation of the external and internal iliac lymph nodes (aqua) and shows that extension to the paramtetrial nodal regions (arrows in (a)) can be confluent with obturator and medial internal iliac node lymph node regions. (c) The lymph node CTV is countoured (green). (d) The final PTV (red) includes the nodal regions and a 1–1.5 cm margin around the cervical tumor/uterus, allowing partial bladder and rectal sparing. (e) A coronal view of the total PTV shows the lymph node CTV, cervical tumor/uterus GTV. The vagina is well delineated using a dry tampon

Fig. 15.7
Contouring of the postoperative target: vaginal tissues. (a) The vaginal tissues can extend well above the vaginal markers or tampon (arrows). The upper extent of the vaginal tampon is visible (middle arrow in a); (b) shows a more superior section. (c) The vaginal cuff is contoured (pink). (c) Coronal view shows the full superior extent of the vaginal tissues; bowel is displaced from the target by bladder (yellow) filling on the right, but close to the vagina on the left
If IMRT is used, two scans, one with full and one with empty bladder, are highly recommended, particularly in postoperative patients. This allows delineation of an internal target volume (ITV) accounting for interfractional organ motion from random organ motion and variable bladder and rectal filling (Chan et al. 2008; Taylor and Powell 2008). Full/empty bladder scans are discussed in more detail in Sect. 2.4.2.
15.1.4.2 Target Volume Delineation
Guidelines for target delineation have been developed by the RTOG (Lim et al. 2011; Small; Small et al. 2008) and are used in RTOG and GOG studies.
The primary tumor GTV includes the cervical tumor and parametrial and vaginal tumor extensions (if MRI is used). If CT is used, which cannot differentiate tumor from the normal uterus, the entire uterus may have to be delineated as GTV, as well as CT evidence of parametrial and vaginal tumor extensions (Fig. 15.6).
The primary tumor CTV includes the cervix (if not already included in the GTV), uterus, parametria, ovaries, and a vaginal margin of at least 2 cm distal to the cervix or lowest vaginal tumor extent based on imaging evidence and per implanted markers (Fig. 15.6d). The boundaries of the parametria extend from the upper border of the fallopian tube superiorly to the pelvic floor muscles inferiorly and the posterior wall of the bladder anteriorly to the mesorectal fascia and uterosacral ligaments posteriorly. Separate delineation of the parametria is challenging on CT and is not mandatory because the PTV usually encompasses the intervening tissues between the uterus and the lymph node target at the pelvic side wall (Fig. 15.6b). If uterosacral ligaments are involved by palpation or imaging, the mesorectum and perirectal lymph nodes should be included.
In patients with known involved lymph node(s), the lymph node GTV contour encompasses the involved node on CT or MRI. Contrast CT to characterize the involved nodes by internal architectural features and/or necrosis or close consideration to architectural MRI features (Sect. 1.3.2) can be helpful. Coregistration of PET and PET/CT (even a diagnostic scan) is most useful in identifying and localizing the involved node(s). However, the delineation should be based on its morphologic geometry and margin on the CT or MRI.
The lymph node CTV contour encompasses the iliac vessels and pelvic lymph nodes, including obturator, external iliac, internal iliac, and common iliac lymph nodes (Figs. 15.2, 15.3, 15.4, 15.5, and 15.6) and 0.7 cm of the perinodal tissue for uninvolved and 1.5 cm for involved lymph nodes. Psoas and piriformis muscles at the pelvic wall and bone are excluded if the lymph nodes are uninvolved. The intraperitoneal small bowel is excluded (Figs. 15.2, 15.3, 15.4, and 15.5). Surgical clips and seromas (Mayr et al. 2011) are included where applicable. The superior extent of the lymph node CTV is typically 0.7 cm below the L4/L5 interspace (so that the PTV, which adds 0.7 cm, extends to the L4/L5 interspace).
However, the traditional 2D-based landmark of L4/L5 has been challenged as the upper border of the common iliac nodes. Significant viability of the aortic bifurcation has been found, and 40 % of common iliac lymph nodes are reported to be located above the L4/L5 interspace (Marnitz et al. 2006). Therefore, the superior border of the lymph node target should be individualized. If high common iliac lymph nodes are involved and no lymph node dissection has been performed, inclusion of the para-aortic lymph nodes should be considered. The inferior extent of the nodal target is just proximal to the inguinal canal, which represents the lower extent of the external iliac nodes. The presacral lymph nodes are included by contouring a 1–2 cm margin of tissue anterior to the sacrum from the sacral levels of S1–S3 (Figs. 15.4 and 15.5); this volume also includes the insertion of the uterosacral ligaments.
For the PTV, an additional margin of 1.5–2 cm is added to the CTV, which varies based on the degree of image guidance and modality (IMRT vs. 3D CRT) used. The distal vaginal margin adds 1 cm to the vaginal PTV, resulting in a total of 3 cm margin from the GTV. The sum of the primary tumor PTV and lymph node PTV results in a confluent final PTV that encompasses the nodal regions, tumor, uterus and parametria, uterosacral ligaments, and presacral lymph nodes (Fig. 15.6).
For the postoperative target, which is described in more detail in Sect. 2.4.2, vaginal, paravaginal, and parametrial CTVs are contoured. Delineation of the lymph node CTV follows the same principles as for intact cervical cancer.
15.1.4.3 Critical Normal Tissue Delineation
Normal structures including the rectum (up to the rectosigmoid junction), bowel including the large bowel above the rectum, and intraperitoneal contents (small bowel and mesentery) within 5 cm above the upper border of the target volume, as well as the bladder and femoral heads, are contoured.
15.2 Endometrial Cancer
15.2.1 Anatomy, Pathology
Pathology
The most common histology is adenocarcinoma; less common histologies include adenosquamous, squamous cell, and mucinous carcinoma. Histologies that infer a worse prognosis and have altered routes of spread include carcinosarcomas, papillary serous carcinomas, and clear cell carcinomas.
The uterus derives its blood supply from several sources that form a rich network of anastomosing vessels. The primary blood supply is from the uterine artery, an anterior branch of the internal iliac artery. The uterine artery enters the uterus after crossing the ureter at the isthmus/level of the internal os. The ovarian artery enters at the level of the fundus through the ovarian ligament. Vaginal arteries anastomose with the uterine vessels inferiorly.
Lymphatic vessels form a rich subserosal plexus that drains from four trunks. The more superior vessels travel through the broad ligament, parallel to the fallopian tube, and drain to the external iliac nodes. More inferiorly, the vessels cross through the broad ligament to the nodes at the bifurcation of the common iliac into the internal and external iliac vessels. At the junction of the fallopian tube and the uterine body are trunks that may pass directly to the para-aortic lymph nodes through the ovarian pedicle. Lymphatics near the round ligament can pass directly along the ligament to the femoral lymph nodes, although this is rare.
15.2.2 Routes of Spread
Endometrial carcinomas initially spread locally, traveling along the mucosa or invading into the myometrium and at times into the cervix. Direct extension into the parametria, bladder, or rectum can occur as can peritoneal spread via the fallopian tubes or through direct serosal extension. Hematogenous spread occurs primarily to the lung and liver.
A landmark surgicopathologic study was published by the Gynecologic Oncology Group and to this day remains the standard work on spread of stage I and occult stage II endometrial carcinoma (Creasman et al. 1987). It demonstrated that the likelihood of spread outside the uterus depends on a combination of factors including grade and depth of myometrial invasion. Overall, in those patients thought to have disease confined to the uterus, 22 % had extrauterine disease including 11 % with pelvic and/or para-aortic disease, 12 % with positive abdominal cytology, 5 % with adnexal involvement, and 6 % with gross peritoneal disease. It is important to note, however, that the more serious prognosis and different routes of spread seen by papillary serous and clear cell carcinoma were not recognized at the time of this study, and those tumors were not separated and evaluated independently. Unlike cervical cancer, lymphatic spread is not limited to a strictly sequential pattern from pelvic to para-aortic lymph nodes, but can occur directly to the para-aortic nodes through the lymphatics along the ovarian vessels, particularly when extensive tumor is present in the upper corpus or fundus.
Tumors with more aggressive histologies tend to metastasize to the lymph nodes and peritoneum, including the upper abdomen, more commonly, although differentiating the behavior of these tumors from aggressive grade 3 tumors is controversial (Alektiar et al. 2002).
Contemporary surgical management includes evaluation of the abdominopelvic cavity with targeted biopsy, collection of peritoneal fluid for cytology, and extrafascial hysterectomy and bilateral salpingo-oophorectomy.
Lymph node dissection is now predicated on risk of nodal spread. Distally, the dissection extends to the circumflex iliac vein and proximally to the bifurcation of the aorta into the common iliac vessels. Laterally, the dissection extends to the genitofemoral nerve that runs along the pelvic sidewall and medially to the superior vesical artery. The most posterior landmark is the obturator nerve. If the intention is to sample the para-aortic nodes, the dissection extends cephalad either to the inferior mesenteric artery (IMA) or the renal vessels. Two large randomized trials did not demonstrate an improvement in survival with surgical staging (Group 2009; Benedetti Panici et al. 2008), and only 30–40 % of patients have formal dissection in the United States (Sharma et al. 2011). The outlines of the dissection should reflect the target for irradiation if it is to substitute for nodal treatment or if added for potential residual disease postoperatively.
Familiarity of the nodal risk based on uterine risk factors such as depth of invasion, grade, and lymphovascular space invasion is essential to assist in the decision to recommend pelvic irradiation in the absence of nodal dissection. What determines an adequate level of risk is debatable, but generally the chance of serious complications from irradiation such as chronic bowel or bladder complaints or lymphedema is reported in the 2–10 % range (van Creutzberg et al. 2000; Keys et al. 2004).
15.2.3 Imaging Modalities, Surgical–Pathology Correlation
Available imaging modalities have limitations in detecting spread of disease, as spread in most patients is microscopic. Local/regional staging is commonly performed utilizing CT with and without intravenous contrast and with bowel contrast. CT has been reported to have an accuracy of 70–80 % for evaluation of nodal disease based on size. Accuracy improves to 93 % with a cutoff value of 1 cm (Hawnaur 1993; Hardesty et al. 2001; Bellomi et al. 2005), and CT is the main modality to delineate lymph node regions and normal structures.
In patients treated for intact endometrial cancer, MRI is excellent in assessing the extent of myometrial and cervical invasion and extrauterine extension (Shin et al. 2011; Cade et al. 2011; Whittaker et al. 2009), which may determine treatment regimens and the decision for brachytherapy alone versus combined external plus brachytherapy. In addition, response of the tumor to external radiation can be assessed to individualize brachytherapy planning.
The (18F)PET/CT has demonstrated a 78 % sensitivity and a 93 % negative predictive value. An ongoing GOG study is evaluating the role of PET/CT in cervical and endometrial carcinoma (Signorelli et al. 2009). Magnetic resonance imaging (MRI) has demonstrated a 75–90 % accuracy rate in assessing nodal spread and invasion into the cervix or myometrium (Rockall et al. 2005; Messiou et al. 2006).
15.2.4 Target Delineation
The principles of target delineation for definitive radiation to the intact uterus follow that of cervical cancer. As primary surgical management is far more common than definitive radiation for uterine cancer, this chapter will focus on largely delineation of the postoperative target.
Defining the target for postoperative pelvic radiation has been the subject of investigation and has sparked national trials (RTOG study 0418) and an atlas (http://www.rtog.org/CoreLab/ContouringAtlases/GYN.aspx) for the postoperative treatment of cervical or uterine carcinomas (Small et al. 2008; Jhingran et al. 2012).
15.2.4.1 Simulation Procedure
Computed tomography should be performed, preferably with intravenous contrast. Bowel contrast can be helpful as well, as long as dosimetric adjustments of the high-density contrast in the bowel are made, which can affect dose calculations (Williams et al. 2002). Consideration should be given to a bowel regimen to minimize stool and gas prior to simulation, as excessive distension displaces the vaginal target anteriorly. If the patient subsequently experiences diarrhea, posterior movement of the vagina can lead to exclusion of the target from the PTV (planning target volume). If excessive rectal distention is noted at the time of simulation, repeat scanning after evacuation or bowel prep may increase target reproducibility. Alternatively, including more of the anterior rectal wall in the CTV (clinical target volume) can be considered, but without image-guided radiation therapy, there is the concern that alteration of the rectal filling will lead to excessive variability in vaginal position.
15.2.4.2 Target Volume Delineation for Postoperative Endometrial Cancer
The postoperative CTV should include the draining lymph nodes as outlined above including the common, external, and internal iliac vessels with a 0.7 cm margin with the upper border as discussed for cervical cancer (Sect. 1.4.2) typically 0.7 cm below the L4/L5 interspace. Contouring of the presacral region to the bottom of S3 should be included for patients with cervical stromal invasion. Inferiorly, the nodal contours will split into a right and left contour, and when the internal iliac vessels are more difficult to visualize, the contours should extend from the external iliac node region anteriorly to the piriformis muscle posteriorly. The nodal CTV continues until the level of the femoral heads, just before the external iliacs exit the pelvis. For postoperative radiation, there is no GTV.
An internal target volume (ITV) is recommended by RTOG 0418 to account for motion of the vaginal structures with variable bladder filling. This is accomplished by performing the simulation CT with both a full and an empty bladder, contouring the CTV and merging the vaginal CTVs, thereby including the vagina and vaginal cuff with both scenarios. Care should be taken to include all vaginal tissue which at times can extend superiorly to the vaginal marker. The lateral extent of the vaginal CTV should extend to the pelvic musculature, and the upper 3 cm of the vagina should be contoured, or alternatively the CTV should be extended to 1 cm above the bottom of the obturator foramen, whichever is lower.
For the lymph node CTV, as for cervical cancer, the typical volume expansion from the vessels and lymph nodes is 0.7 cm and excludes the bone and the psoas and piriformis muscles but should include any visible lymph nodes or pertinent clips from surgical dissection (Messiou et al. 2006). At times, large lymphoceles are noted on the postoperative CT, and these should be included in the CTV. However, with a negative dissection, the risk of tumor contamination is low, and it should be noted that inclusion of the lymphoceles into the CTV will decrease normal tissue sparing. Anterior to the vertebral bodies and the sacrum, the CTV should include 1.5 cm of tissue while excluding the bone. It is not necessary to extend the CTV into the sacral foramina, but caution should be used to include the potential space medial to the psoas muscle and lateral to the vertebral body (Fontanilla et al. 2013).
The para–aortic CTV is contoured if the para-aortic nodes have not been sampled or if involved nodes are found. The vertebral interspace of the level T11/T12 vertebra has been historically the upper extent of the treatment volume, for surgical dissection extends to the inferior mesenteric artery or renal vessels. If concurrent chemotherapy is given, consideration should be given to lowering the fields below the T11/T12 level. The cisterna chyli lies posterior to the aorta just superior to the renal vessels, anterior to the first or second lumbar vertebra.
For the PTV, variability exists in recommendations for the PTV margin. RTOG 0418 recommended a 0.7 cm margin on the nodal and vaginal CTV/ITV, but earlier studies, especially those without an ITV, used margins of 1 cm (Lujan et al. 2003; Georg et al. 2006). Without an ITV, consideration should be given to daily image guidance or larger PTV margins. Motion studies have demonstrated differential movement laterally and anteriorly/posteriorly, and target motion has ranged from 1.5 to 2.3 cm, primarily due to bladder filling and emptying (Jürgenliemk-Schulz et al. 2011; Harris et al. 2011). Positional change with rectal filling/emptying has been variably reported.
15.2.4.3 Target Volume Delineation for Intact Endometrial Cancer
Treatment with external beam and/or brachytherapy for medically inoperable uterine carcinoma has not received the same scrutiny as medically inoperable uterine cancer is uncommon. However, guidelines for external beam irradiation generally mimic those recommended for intact cervical carcinoma.
For brachytherapy in intact uterine cancer, several different brachytherapy systems are available for intact uterine cancer, including single tandem, dual tandem (Y-tandem), triple tandem, and Heyman packing (Nag et al. 2000a; Coon et al. 2008; Bauer et al. 1991; Weitmann et al. 2005). In addition, both low-dose-rate and high-dose-rate deliveries are acceptable.
The system used should be chosen based on the ability to cover the tumor and full uterine wall and vaginal thickness (Mock et al. 1998). With new MRI imaging, the ability to discern the location and depth of tumor invasion is far more sophisticated (Shin et al. 2011; Cade et al. 2011; Whittaker et al. 2009). The choice between ovoids and cylinder for coverage of the vagina depends on preference, unless there is vaginal extension below the dosimetric coverage of the ovoids. In that instance, a vaginal cylinder should be used for full coverage. If gross disease is present, it is prudent to mark the inferior extent of disease with a radiopaque gold marker to assure full coverage, especially if external beam irradiation is given initially as the tumor may regress prior to the brachytherapy boost. Historically, with 2D dosimetry, the dose was prescribed 2 cm from the uterine tandem. Ideally, 3D imaging should be used, and assurance of coverage of the entire uterine serosa and targeted vagina should be covered by the prescription isodose line. Detailed suggestions on treatment, coverage, dose specification, and optimization have been published by the American Brachytherapy Society (Nag et al. 2000b).
Vaginal cuff brachytherapy alone has become increasingly more common for postoperative endometrial carcinoma. Cylinders and ovoids have been used with advantages and disadvantages to both (Kim et al. 2002). In addition, there are molds, shielded cylinders, and multichannel applicators available for customization of dosimetry. Most importantly, the largest vaginal cylinder should be used to maximize dosimetry and avoid air gaps between the cylinder and the mucosa and to avoid underdosing the target (Richardson et al. 2010). Different fractionation schemes and lengths of the vagina treated are used, and a summary of choices with detailed recommendations for postoperative cuff treatment and treatment of cuff recurrences has been published by the American Brachytherapy Society and recently updated (Small et al. 2012, 2005). Most physicians choose to treat only the upper portion of the vagina adjuvantly and treat the entire length of the vagina only in cases of positive margins, recurrent disease, or with other high-risk pathological findings. For gross disease, the thickness of tumor should not exceed 5 mm if intracavitary brachytherapy is planned. If this thickness is exceeded, it is best to treat with interstitial therapy according to established guidelines (Beriwal et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree