Gynecologic Malignancies
Arno J. Mundt
Loren K. Mell
Catheryn Yashar
Introduction
Gynecologic cancers arise from organs throughout the female reproductive tract, including the ovaries, uterus, cervix, vagina and vulva. Gynecologic cancers represent the fourth most common malignant tumors diagnosed in women in the United States each year, with approximately 83,000 cases expected in 2010 (1). Worldwide, gynecologic cancers represent approximately 20% of all cancers, with nearly one million cases diagnosed per year (2).
Radiation therapy (RT) occupies an important role in the treatment of nearly all gynecologic malignancies. Radiation is commonly used as definitive treatment in many early stage patients and in conjunction with surgery and/or chemotherapy in women with locally advanced disease. It is also frequently used to palliate patients when cure is no longer possible.
This chapter provides an overview of the role of RT in the treatment of gynecologic malignancies, with a focus on the planning of various radiotherapeutic approaches used in these patients, including external beam RT and brachytherapy. Novel technologies including intensity-modulated RT (IMRT) and image-guided RT (IGRT) as well as their emerging application in the treatment of these cancers are also discussed.
Radiotherapeutic Management
Cervical Cancer
Radiation is commonly used in the treatment of nearly all stages of cervical cancer. Select early (microscopic) tumors (Stage IA) are treated primarily with surgery; however, when such patients are unable to undergo surgery due to advanced age and/or significant comorbidities, RT can be used and is associated with excellent results (3). Early stage patients with gross disease (stages IB–IIA) are managed well with either radical surgery or definitive RT, with cure rates exceeding 80% following either approach (4).
The choice of surgery versus radiation in early stage cervical cancer depends on a number of factors, including patient age, comorbidities and various tumor characteristics. Older women, particularly those with multiple comorbidities, are generally treated with RT, whereas younger women receive surgery. A common reason for favoring surgery in young women is the ability to preserve their ovarian function. However, it may be possible to preserve ovarian function in pre-menopausal patients by performing an ovarian transposition prior to RT (5). Another oft-stated reason for favoring surgery in young women is the commonly held belief that sexual function would be less adversely affected. However, prospective quality-of-life analyses have found equivalent sexual function following surgery compared to RT (6). Moreover, new approaches have been developed to address sexual dysfunction in irradiated women (7).
In general, RT is recommended over surgery in early stage cervical cancer patients as lesion size and vaginal involvement increase. As tumor diameter exceeds 4 cm, there is increased likelihood of tumor spread to surrounding organs and regional lymph nodes, necessitating the need for adjuvant RT following surgery. Randomized trials conducted by the Gynecologic Oncology Group (GOG) and other cooperative groups have found that postoperative RT is beneficial in many cervical cancer patients following surgery. GOG 92 noted an improved 2-year recurrence-free survival rate (88% vs. 79%, p = 0.008) comparing adjuvant RT versus no further therapy in node negative patients with high risk features (deep stromal invasion, bulky primary disease and/or lymphovascular invasion [LVI]) (8). GOG 109 compared adjuvant pelvic RT versus pelvic RT plus chemotherapy in women found to have involved pelvic nodes, parametrial invasion and/or positive margins and noted a superior 4-year overall survival (81% vs. 71%, p = 0.007) with the combined approach (9). Whether concomitant chemoradiotherapy is superior to RT alone in node negative patients with high-risk features is currently being evaluated by GOG 263, whereas the benefit of adjuvant chemotherapy following chemoradiotherapy in high-risk node positive patients is the subject of GOG 0724.
While a treatment option in early stage patients, radiation has long been the cornerstone of treatment in cervical cancer patients with locally advanced (stage IIB–IVA) disease. In these women, radiation is combined with concomitant chemotherapy in light of multiple prospective randomized trials demonstrating a survival advantage to the combined approach (10,11,12). Surgery is typically not utilized in patients with locally advanced disease, albeit
some investigators have advocated pelvic exenteration in cases with bladder and/or rectal invasion (stage IVA) (13). Metastatic (Stage IVB) patients may also undergo RT, particularly those with a good response to chemotherapy or those requiring palliative treatment due to uncontrolled vaginal bleeding.
some investigators have advocated pelvic exenteration in cases with bladder and/or rectal invasion (stage IVA) (13). Metastatic (Stage IVB) patients may also undergo RT, particularly those with a good response to chemotherapy or those requiring palliative treatment due to uncontrolled vaginal bleeding.
Definitive RT in cervical cancer is administered with a combination of pelvic RT and brachytherapy, except in earliest stage patients in whom brachytherapy alone is sufficient (3). Early stage patients (stage IB1) is standardly treated with radiation as there are currently no randomized trials demonstrating improved outcomes with the addition of chemotherapy. Early stage patients with bulky (>4 cm) tumors (stage IB2) are treated with a combination of pelvic RT and chemotherapy (14), with or without an adjuvant hysterectomy (15). Hysterectomy increases local control but does not alter survival. When delivered adjuvantly, most patients receive pelvic RT with or without chemotherapy. In women with locally advanced disease, pelvic fields are also used, except in those with documented paraortic lymph node involvement in whom extended field RT (EFRT) is administered. In the past, there was considerable interest in prophylactic para-aortic RT in locally advanced patients, following the superior survival rates reported on the Radiation Therapy Oncology Group (RTOG) trial using this approach (16). Today, however, EFRT is rarely used without evidence of paraortic involvement, except at some centers in woman with involvement of common iliac lymph nodes. Pelvic fields are extended to include the inguinal lymph nodes in patients with lower vaginal involvement and additional external beam RT is often delivered in patients with significant parametrial involvement and/or gross nodal disease.
Brachytherapy is commonly delivered in conjunction with pelvic RT in cervical cancer patients undergoing definitive treatment. In the adjuvant setting, brachytherapy is less commonly performed, except when patients are treated preoperatively. If brachytherapy is prescribed, most patients receive intracavitary brachytherapy (ICB). However, at some centers, interstitial brachytherapy is favored in women with unfavorable anatomy and/or significant parametrial involvement. See section on “Radiotherapeutic Techniques” for a full discussion of the various RT techniques used in cervical cancer patients.
Uterine Cancer
Although radiation has traditionally been delivered prior to surgery in many uterine cancer patients (17), the majority of patients today undergo upfront surgery, consisting of total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO), with RT delivered postoperatively to select patients based on pathologic features in the surgical specimen. In the United States, many uterine cancer patients undergo extended surgical staging (ESS) at the time of surgery as well, consisting of pelvic and para-aortic lymphadenectomies, despite the results of two randomized trials questioning its benefits in terms of survival (18,19).
The most common pathologic features used to determine the need for adjuvant RT in early stage (stage I–II) uterine cancer are tumor grade, histology, depth of myometrial invasion (MI), LVI, and cervical involvement. Some investigators also use tumor size and lower uterine segment (LUS) involvement, albeit the prognostic significance of such features, particularly in the absence of other more established adverse features, remains unclear (20). Multiple randomized trials have been performed by the GOG and other groups demonstrating that adjuvant RT in early stage uterine cancer patients with adverse pathologic features significantly reduces locoregional failure (21,22,23,24). Whether it improves survival, however, remains a matter of intense debate, despite the results of two large Surveillance Epidemiology and End Results (SEER) studies suggesting a survival benefit in most patients (25,26).
At many centers today, low-risk early stage uterine cancer patients (stage I, grade 1, no or minimal MI) are treated with surgery alone, while those with intermediate risk factors (grade 3 with superficial MI or grade 1 to 2 with deep MI, especially combined with advanced age and LVI) receive adjuvant RT. In the latter group, pelvic RT is currently reserved for patients who do not undergo ESS and vaginal brachytherapy is delivered in those who do (provided all nodes are negative)—a trend that appears to be increasing in the United States (27). It may be possible, however, to treat select intermediate risk patients with adjuvant vaginal brachytherapy alone even when ESS is not performed (28). In the adjuvant setting, either pelvic RT or vaginal brachytherapy are delivered. While commonly delivered together in the past, the routine use of both appears to only increase the risk of serious toxicity without improving local control and thus should be avoided whenever possible (29).
The optimal approach in high-risk early stage uterine cancer patients (deep MI and grade 3 disease, cervical stromal invasion, LVI) remains controversial. In Europe, the Postoperative Radiotherapy for Endometrial Carcinoma (PORTEC)-3 trial is comparing pelvic RT versus pelvic RT plus concomitant and adjuvant chemotherapy in these patients. In the United States, GOG 0249 is randomizing patients to pelvic RT versus vaginal brachytherapy plus chemotherapy.
In early stage uterine cancer patients unable to undergo surgery due to advanced age and/or multiple co-moribidities radiation can be used with curative intent and typically consists of a combination of brachytherapy with or without pelvic RT in grade 3 patients and brachytherapy alone in those with grade 1 to 2 disease (30). Pelvic magnetic resonance imaging (MRI) may be helpful in these women to evaluate MI and extra-uterine spread.
In stage III and IV uterine cancer, the standard approach has been for many years surgery followed by adjuvant RT, using a variety of techniques including pelvic RT, EFRT
and whole abdominal RT (WART). Historically, select locally advanced patients with positive peritoneal cytology received intraperitoneal radioactive phosphorus (32P) (31). Outcomes varied considerably in stage III–IV patients depending on the type and number of extrauterine sites involved, with the best results seen in patients with isolated adnexal involvement (32) and poorer outcomes in those with involvement of multiple extra-uterine sites, nodal involvement and/or residual disease (33).
and whole abdominal RT (WART). Historically, select locally advanced patients with positive peritoneal cytology received intraperitoneal radioactive phosphorus (32P) (31). Outcomes varied considerably in stage III–IV patients depending on the type and number of extrauterine sites involved, with the best results seen in patients with isolated adnexal involvement (32) and poorer outcomes in those with involvement of multiple extra-uterine sites, nodal involvement and/or residual disease (33).
Today, WART is rarely used in the United States following the publication of the GOG 122 trial randomizing stage III–IV patients to either WART or chemotherapy which noted a superior 5-year survival (55% vs. 42%) with adjuvant chemotherapy but inferior pelvic control (34). However, the question remains whether adjuvant RT has a role in conjunction with chemotherapy. Patterns of failure studies suggest that it does, given the high risk of loco-regional recurrence in women undergoing surgery and chemotherapy (35). At many centers today, stage III and select stage IV patients are treated with chemotherapy combined with limited volume RT (pelvic RT, EFRT and/or vaginal brachytherapy based on pathologic features), an approach known as “tumor volume-directed” RT, which was used in the GOG 184 trial (36). Of note, GOG 258 is currently randomizing locally advanced patients to chemotherapy versus chemoradiotherapy. The results of this important trial will help define the role of RT in these patients.
The role of RT in the treatment of patients with unfavorable histologies—notably papillary serous and clear cell tumors—is controversial. However, RT may help reduce the risk of locoregional recurrence in such patients who receive adjuvant chemotherapy following surgery (37). Patients with early stage uterine sarcomas, except those with low-grade endometrial stromal sarcoma, have traditionally been treated with adjuvant pelvic RT. Locally advanced sarcoma patients are treated at many centers with adjuvant chemotherapy without RT, due to the superior outcomes in patients undergoing chemotherapy compared to WART on the GOG 150 trial (38). Nonetheless, adjuvant RT still remains a common treatment of choice in these patients, particularly in elderly women and others who are unable to undergo adjuvant chemotherapy. See section on “Radiotherapeutic Techniques” for a full discussion of the various RT techniques used in patients with uterine cancer.
Ovarian Cancer
For many years, RT occupied an important role in the treatment of ovarian cancer. Following upfront surgery, consisting of an omentectomy, TAH-BSO, peritoneal sampling and cytoreduction of extra-ovarian disease throughout the abdomen and pelvis, and often pelvic and para-aortic lymph node dissection, adjuvant RT was routinely delivered in the form of intraperitoneal 32P in high-risk early stage disease and WART in locally advanced disease patients.
Several randomized GOG studies have compared adjuvant 32P versus chemotherapy (melphalan or cisplatin-cytoxan) in early-stage high risk disease (stages IA–B grade 3, stages IC–II) and found no differences in either relapse-free or overall survival rates (39,40). Moreover, in GOG 7602, chemotherapy was associated with higher toxicity rate compared to RT, including a higher rate of second malignancies (39). Despite these favorable results, few patients receive intraperitoneal 32P today, apart from its use at some centers in select elderly high-risk early stage patients unable to receive chemotherapy, partly due to the development of peritoneal adhesions in some patients.
In locally advanced ovarian cancer patients who were optimally cytoreduced (previously defined as <2 cm residuum remaining following surgery), WART was long considered the standard adjuvant approach at many centers throughout the world. Published long-term outcomes have been highly favorable in such patients treated with WART, particularly in those with microscopic or no residual disease after cytoreductive surgery (41,42). Investigators at Stanford University reported a 15-year relapse-free survival rate of 50% in stage I–III optimally cytoreduced patients undergoing adjuvant WART (41). Despite these favorable results and even a prospective phase III MD Anderson Hospital randomized trial demonstrating identical survival rates following WART or chemotherapy (43), adjuvant WART has been largely abandoned at most centers today—at least in the United States. The standard adjuvant approach is currently combination chemotherapy. Unfortunately, a randomized trial comparing WART and chemotherapy regimens popular today will most likely never be performed.
Although no longer used alone following surgery, the question remains whether adjuvant adjuvant RT has a role in conjunction with chemotherapy and surgery in ovarian cancer. Several prospective trials have evaluated the role of “consolidative” RT in stage I-III patients following upfront surgery and adjuvant chemotherapy (44,45). No benefit was seen using intraperitoneal 32P compared to observation in a GOG trial focused on stage I–III patients with microscopic residual disease following surgery and chemotherapy (44). In contrast, the Swedish-Norwegian Ovarian Cancer Study Group trial compared WART versus cisplatin-doxorubicin-epirubicin versus observation in stage III patients achieving a pathologic complete response and noted 5-year progression-free survivals of 56%, 36% and 35% in the WART, chemotherapy and observation groups, respectively (45). Although favorable results have been reported combining adjuvant RT with modern taxane-based adjuvant chemotherapy (46), whether adjuvant RT is truly beneficial in this setting remains unclear.
RT is occasionally used in the management of patients with non-epithelial ovarian cancers. Given its exquisite radiosensitivity, ovarian dysgerminoma may be treated with RT; however, in these patients as well, the standard practice today is adjuvant chemotherapy, particularly in
those desiring fertility-sparing treatment. As in other gynecologic cancers, RT has an important role in the palliative treatment of ovarian cancer patients (47). See section on “Radiotherapeutic Techniques” for a discussion of the various RT techniques used in ovarian cancer.
those desiring fertility-sparing treatment. As in other gynecologic cancers, RT has an important role in the palliative treatment of ovarian cancer patients (47). See section on “Radiotherapeutic Techniques” for a discussion of the various RT techniques used in ovarian cancer.
Vulvar Cancer
The treatment of vulvar cancer at most centers today consists of upfront surgery, typically radical vulvectomy or radical wide local excision in select patients with small well-lateralized tumors (48), with RT delivered adjuvantly in patients with high-risk features including LVI, tumor invasion >5 mm, surgical margins < 8 mm, grade 3 disease, and microscopic positive margins (49,50). Most patients also undergo bilateral inguinofemoral dissections, particularly those found to have tumor invasion >3 mm, LVI and/or high-grade disease, due to their high risk of nodal involvement.
Although commonly used in many other gynecologic cancers, prophylactic nodal irradiation is rarely used to treat clinically negative regional lymph nodes in vulvar cancer. This practice is based on the GOG 88 trial which compared prophylactic inguinofemoral RT versus lymphadenectomy in clinically node negative patients and found a significantly higher rate of recurrence (p = 0.03) and death (p = 0.04) in the RT group (51). See section on “Radiotherapeutic Techniques” for a critique of this influential study.
In women with locally advanced but resectable disease, surgery is typically performed followed by adjuvant radiation or chemoradiation. Over 20 years ago, the GOG completed a landmark trial (GOG 37) comparing adjuvant RT versus pelvic node dissection in patients found to have involved inguinal nodes at the time of surgery (52). A significantly higher survival rate was noted in the RT group, with the greatest benefit seen in women with clinically suspicious and/or ≥2 pathologically involved nodes. Moreover, adjuvant RT significantly reduced the risk of recurrence in the inguinal nodes (5% vs. 24%, p = 0.02). This trial established adjuvant RT as the treatment of choice in these patients.
In unresectable vulvar cancer patients, RT has been used preoperatively with promising results (53,54). Considerable interest has been focused on further augmenting these results by combining RT with chemotherapy (50,55,56). GOG 101 evaluated preoperative chemoradiotherapy in locally advanced unresectable patients and found that nearly all patients (97%) became resectable, with only 16% ultimately failing locally (57).
Adjuvant RT in vulvar cancer is delivered using a variety of techniques, ranging from small electron fields directed solely at the primary site to generous fields encompassing the primary, pelvis plus one or both groins. The most common approach, however, is pelvic-inguinal irradiation, delivered with or without a midline block. Brachytherapy has a limited role in vulvar cancer, apart from patients with a positive vaginal margin or in medically inoperable patients in whom high doses are required to control the primary tumor. However, at most centers, high dose central boosts are delivered in such patients using external beam techniques. A description of the various RT techniques used in vulvar cancer is provided below (see section on “Radiotherapeutic Techniques”).
Vaginal Cancer
The treatment of choice in all stages of vaginal cancer is definitive RT. Select early stage patients, for example, those with small-volume disease limited to the upper vagina, however, can be treated with a variety of surgical approaches, including partial or radical vaginectomy (58).
Early stage vaginal cancer patients typically receive brachytherapy alone or is combined with external beam when tumors invade into the paravaginal tissues (59,60). Some investigators advocate the use of combined external beam and brachytherapy even in patients without paravaginal invasion (61). Overall, definitive RT is associated with excellent outcomes in most early stage vaginal cancer patients, particularly in those with stage I disease in whom locoregional control rates exceed 85% (60). Patients with locally advanced disease undergo pelvic RT and brachytherapy (59,60,61). At some institutions, chemotherapy is delivered concomitantly in these patients in an effort to improve tumor control and potential survival extrapolation from cervical cancer studies. Given the low number of vaginal cancer patients diagnosed each year, it is unlikely that randomized trials evaluating such approaches will ever be successfully conducted.
Brachytherapy in early stage vaginal cancer typically involves ICB, but in women with >0.5 cm tumor invasion or thickness, interstitial brachytherapy is recommended. Some investigators favor interstitial brachytherapy combined with ICB even in patients with superficial tumors to reduce the total vaginal dose and, in distal vaginal tumors, the rectal dose (61). If external beam is also delivered, vaginal cancer patients receive either pelvic irradiation, or in cases involving the lower one-third of the vagina, pelvic-inguinal RT. See section on “Radiotherapeutic Techniques” for a discussion of the various RT techniques used in patients with ovarian cancer.
Radiotherapy Techniques
External-Beam Therapy
Whole Pelvic Radiotherapy
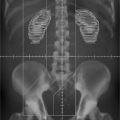
Pelvic RT is used in many gynecologic cancers to irradiate multiple target tissues within the pelvis, including the uterus/cervix (or the postoperative bed), the upper vagina, paracervical/parametrial tissues and the pelvic (internal, external and common iliac) lymph nodes. Pelvic RT fields have traditionally been designed using fluoroscopic simulation based on bony landmarks and are delivered with either two-field (opposed anterior-posterior:posterior-anterior
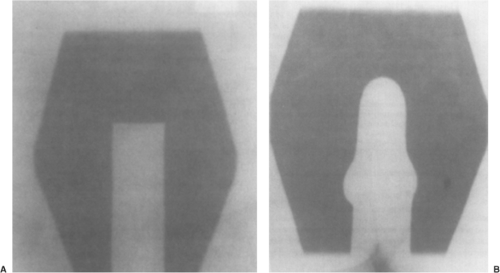
[APPA] fields) or four-field (APPA plus opposed lateral fields) techniques (Fig. 25.1). In recent years, however, most centers have moved away from APPA treatment (62), since four-field approaches allow considerably more normal tissue sparing, notably the anterior small bowel and posterior rectum. Some investigators still favor opposed APPA fields, however, in patients with locally advanced cervical cancer.
[APPA] fields) or four-field (APPA plus opposed lateral fields) techniques (Fig. 25.1). In recent years, however, most centers have moved away from APPA treatment (62), since four-field approaches allow considerably more normal tissue sparing, notably the anterior small bowel and posterior rectum. Some investigators still favor opposed APPA fields, however, in patients with locally advanced cervical cancer.
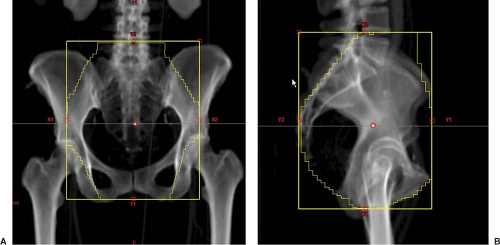 Figure 25.1. Anterior-posterior (AP) (A) and lateral fields (B) in a representative gynecologic cancer patient undergoing whole pelvic radiation therapy. |
For many years, the superior border of the pelvic RT field was placed at the L5-S1 interspace; however, most investigators currently favor the L4–L5 interspace to include the common iliac lymph nodes. It is important to note, however, that the common iliacs in some patients may extend considerably higher, requiring an upper border as high as L2–L3 to ensure their full coverage. The lower pelvic RT border is typically placed at the inferior obturator foramen while the lateral borders are set 1–1.5 cm beyond the pelvic brim. The anterior border of the lateral field is at (or 1 cm anterior) the pubic symphysis to ensure coverage of the external iliac nodes; the posterior border is at the S2–S3 interspace. Previously, the posterior border was commonly set at the S1–S2 interspace; however, concerns were raised about adequate coverage of the attachment of the uterosacral ligament to the sacrum with this approach, particularly in locally advanced cervical cancer patients (63). Customized blocking is added to each field using either cerrobend or multileaf collimation to reduce dose to the surrounding normal tissues including the small bowel and rectum. Oral and rectal contrast may be administered at simulation to aid in the design of these blocks.
Various modifications can be done to the traditional pelvic RT fields, depending on the clinical scenario. In cervical or uterine cancer patients with significant vaginal involvement, the lower border can be extended to the introitus ensuring irradiation of the entire vaginal canal. In such cases, it is helpful to place a radiopaque marker in the vagina at the time of simulation marking the inferior extent of disease. The superior border may be extended superiorly to fully encompass the common iliac lymph nodes, particularly in women with involvement of the external iliac lymph nodes, or may be lowered to the L5-S1 interspace, in patients undergoing ESS and found to have negative pelvic nodes. The posterior border of the lateral field can also be moved posterior to the sacrum in cervical cancer patients with bulky disease, whereas the anterior border may be moved anteriorly in women with an anteverted uterus and/or bulky external iliac nodes.
At many centers, patients undergoing pelvic RT are immobilized and simulated in the supine position. Prone positioning with a “belly board” is favored by some investigators to help reduce the volume of small bowel irradiated. Similarly, at some centers, patients are simulated and treated with a full bladder to displace small bowel. Whatever approach is used, it is important to strive for consistent bladder and rectal filling throughout treatment, given that the volume of these organs may impact on the position of the cervix/uterus (or vaginal cuff) (64,65).
Although pelvic RT fields have traditionally been designed based on bony landmarks, multiple investigators have demonstrated that such an approach may underdose the target tissues and/or inadequately shield surrounding normal tissues in many patients (66,67). Finlay and colleagues assessed the adequacy of nodal coverage of conventional pelvic RT fields in 43 cervical cancer patients (66). Pelvic vessels were contoured following computed tomography (CT)
simulation and used as surrogates for the pelvic lymph nodes. In 41 patients (95%), conventional fields inadequately covered various lymph node groups. Of note, in 24 (56%), conventional fields and blocks were found to be too generous. Others have similarly used three-dimensional (3D) imaging and found that conventional fields and blocking may result in inadequate target coverage in up to 50% of patients, particularly with the placement of the posterior field border of the lateral pelvic field at S2–S3 (67). These results strongly support the design of pelvic RT field using CT simulation—a trend that is growing in the community.
simulation and used as surrogates for the pelvic lymph nodes. In 41 patients (95%), conventional fields inadequately covered various lymph node groups. Of note, in 24 (56%), conventional fields and blocks were found to be too generous. Others have similarly used three-dimensional (3D) imaging and found that conventional fields and blocking may result in inadequate target coverage in up to 50% of patients, particularly with the placement of the posterior field border of the lateral pelvic field at S2–S3 (67). These results strongly support the design of pelvic RT field using CT simulation—a trend that is growing in the community.
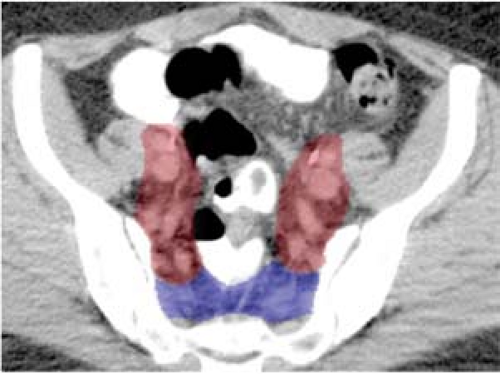
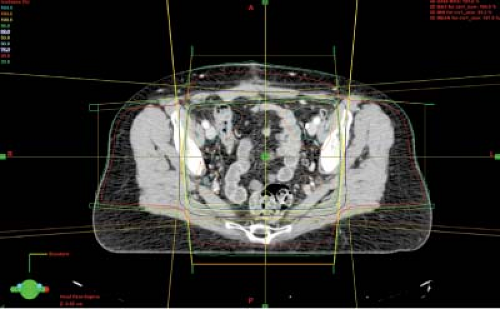 Figure 25.2. Treatment plan with isodose lines in a representative gynecologic cancer patient treated with whole pelvic radiation therapy. |
Conventional pelvic RT fields are typically delivered using moderate-high energy (≥10 MV) photon beams. However, lower energies can be used in select thin patients. Wedges may be added to the lateral fields to reduce “hot spots” in the treatment plan. As example, pelvic RT plan is shown in Figure 25.2. Total doses prescribed typically range from 39.6–50.4 Gy delivered in 1.8–2 Gy/fractions, the higher doses used in patients undergoing external beam alone and lower doses in those treated with both external beam and brachytherapy.
In the past, there was considerable interest in the use of altered fractionation schedules in gynecologic cancer patients, including accelerated hyperfractionated RT as a means of escalating the pelvic radiation dose. However, the RTOG 88-05 trial using this approach in locally advanced cervical cancer patients failed to demonstrate a benefit in terms of tumor control or complications (68). Others have also reported high complication rates using a hyperfractionated approach (69). Moreover, twice daily treatment in an effort to boost the cervical tumor prior to brachytherapy has also been associated with an increased complications (70). In contrast, hypofractionated approaches, for example, 2.5–3 Gy daily fractions (total doses 30–35 Gy) have been used successfully in patients treated palliatively. Higher dose per fraction (up to 10 Gy) palliative regimens have also been explored (71,72).
Various blocks can be added to the conventional pelvic RT field. At some centers, a midline block is utilized allowing a higher proportion of the total dose to be delivered by brachytherapy in cervical cancer patients, typically placed after approximately 20 Gy (73). A midline block may also be placed following brachytherapy in cervical cancer patients with significant parametrial involvement and/or involved pelvic lymph nodes allowing an additional boost to be delivered, typically 10–12 Gy in five to six fractions.
At many centers, midline blocks are often standardized, but at others they may be customized based on the brachytherapy isodose distributions (Fig. 25.3). Wolfson and colleagues performed a survey of GOG institutions and reported that the percentages of centers using standard and customized midline blocks were 76% and 21%, respectively (74). Only 3% utilized step-wedge blocks as popularized by Perez and associates (73). The width of the midline block should fully encompass the colpostats on the anterior radiograph plus a margin since narrow mid-line blocks inadequately shield the ureters and are associated with increased complications (75). The inferior edge of the block should be coincident with the lower border of the pelvic field. However, various superior block edges can be used. In the GOG survey, the upper border of the midline block was set at the top of the pelvic field, the tip of the tandem plus a 1 to 2 cm margin or the level of the sacro-iliac joint by 29%, 38% and 33% of respondents, respectively (74).
IMRT is increasingly being used in the treatment of gynecologic cancers. Investigators at the University of Chicago were the first to compare conventional and IMRT planning in gynecologic cancer patients undergoing pelvic RT and found that IMRT reduced the volume of small bowel irradiated by a factor of two and the volume of both the bladder and rectum irradiated by 23% (76). Subsequently, others have confirmed these results, with reductions up to 70% seen with IMRT planning in terms of the volume of small bowel receiving the prescription dose (77,78,79). More recently, IMRT planning has been shown to be an effective means of reducing the volume of pelvic bone marrow irradiated in patients undergoing pelvic RT—an appealing approach particularly in patients receiving concomitant chemotherapy (80).
Patients undergoing pelvic IMRT are typically immobilized in the supine position and undergo CT simulation with thin (5 mm) slices, although at select centers prone positioning is favored (81). Contrast may be administered to aid in the delineation of the target and normal tissues; intravenous contrast is particularly useful since the pelvic vasculature is used as a surrogate for the lymph nodes in the planning process.
Following simulation, a gross tumor volume (GTV) and clinical target volume (CTV) are contoured on the planning CT scan, based on ICRU 50 guidelines (82). The GTV should include all demonstrable disease, including involved regional lymph nodes. A variety of imaging modalities can be used to aid in target design, with growing attention on positron emission tomography (PET) and MRI (83,84). Investigators at Washington University base their target volume definition on PET and contour a metabolically active tumor volume (MTV), specified at the 40% threshold level (85).
The CTV in most patients undergoing pelvic IMRT consists of the upper half of the vagina, uterus/cervix (if present), parametria, pre-sacral region and pelvic lymph nodes (internal, external and lower common iliac nodes). The most superior extent of the CTV is typically placed 1–1.5 cm inferior to the L4–L5 interspace to account for planning target volume (PTV) expansions. In uterine cancer patients without cervical involvement, the pre-sacral region need not be included. At some centers, a single CTV is drawn, whereas at others several CTVs are delineated. In a postoperative patient, for example, two CTVs may be delineated; the CTVnodes includes the common, external and internal iliac nodes and pre-sacral space, whereas the CTVvagina includes the vaginal cuff and paravaginal/parametrial tissues. At some centers, an integrated target volume (ITV) is generated by fusing empty and full bladder planning CT scans, encompassing contours of the cervix (or vaginal cuff in postoperative patients) on both scans, with patients treated with a full bladder or, at other centers, an empty bladder as maintaining a full bladder has not been shown to be reproducible. Normal tissues are contoured as well, including the small bowel, bladder, rectum and, at some centers, the sigmoid colon. The pelvic bones are used as a surrogate for the pelvic bone marrow in patients undergoing chemoradiotherapy. In the past, only the iliac crests were contoured; however, recent data suggest that the pelvic bones are a better surrogate for the bone marrow in the optimization process (86).
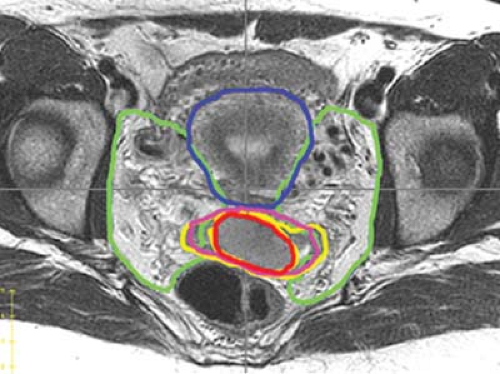
Consensus guidelines regarding CTV design in gynecologic cancer patients undergoing postoperative pelvic IMRT were developed for the RTOG 0418 phase II trial (Fig. 25.4) (87). These guidelines were based, in part, on
work of Taylor et al. who mapped pelvic lymph node regions using iron oxide-enhanced MRI, a method to visualize benign lymph nodes (88). In an analysis of 20 patients using this technique, a modified margin of 7 mm around the major pelvic vessels was found to encompass 99% of the visualized lymph nodes. More recently, consensus guidelines have been developed for cervical cancer patients treated with an intact uterus based on MRI (Fig. 25.5) (89).
work of Taylor et al. who mapped pelvic lymph node regions using iron oxide-enhanced MRI, a method to visualize benign lymph nodes (88). In an analysis of 20 patients using this technique, a modified margin of 7 mm around the major pelvic vessels was found to encompass 99% of the visualized lymph nodes. More recently, consensus guidelines have been developed for cervical cancer patients treated with an intact uterus based on MRI (Fig. 25.5) (89).
The next step in the IMRT planning process involves the expansion of the CTV to generate a PTV, accounting for patient setup uncertainty and organ motion. The optimal PTV expansion remains a matter of debate, particularly in patients with intact cervical cancer in which there may be considerable organ motion (64,65). A reasonable approach is to provide generous expansions around the cervical tumor (definitive patients) and the vaginal cuff (postoperative patients) on the order of 1.5–2 cm. Tighter expansions can be placed around the CTV in the upper pelvis (0.7–1 cm). Daily in-room image-guided RT (IGRT) techniques, notably cone-beam CT (CBCT) imaging, may help ensure adequate coverage of the target tissues on a daily basis (64).
No consensus exists regarding many IMRT planning parameters used in gynecologic cancer patients. At many centers seven to nine equally spaced 6-MV beams are used; however, others favor volumetric arc or tomotherapy approaches. As in conventional RT, the total dose prescribed is a function of the tumor site, stage and treatment volume. Most investigators deliver 45 Gy in 1.8 Gy daily fractions, particularly in women subsequently undergoing brachytherapy. Higher doses (50.4 Gy) can be used in patients treated with pelvic IMRT alone (90). Some investigators are exploring the use of more sophisticated simultaneous integrated boost (SIB) approaches, particularly in women with grossly positive nodes (91).
Given the potential ability to safely deliver higher and potentially more efficacious doses, some investigators have explored using IMRT as a substitute for brachytherapy in cervical cancer (92,93). Investigators at the Princess Margaret Hospital recently presented a case study of a stage IIB cervical cancer patient unsuitable for brachytherapy treated with an IMRT boost (94). Using MRI guidance, a GTV was delineated consisting of the cervix and LUS. The GTV was expanded by a 10 mm margin (7 mm posteriorly) generating the CTV which was subsequently expanded by 5 mm (10 mm anteriorly) to generate the PTV. Six static 6 MV fields were used to deliver 25.2 Gy in 1.8 Gy daily fractions to the PTV. The resultant treatment plan was highly conformal; average doses delivered to 50% of the rectum, bladder and small bowel were 21, 13 and 5 Gy, respectively. Treatment was tolerated well without significant sequelae. Given the paucity of data using IMRT in lieu of brachytherapy, this approach should be considered experimental at the present time and only used in women unable to undergo brachytherapy.
The optimal dose–volume constraints for the PTV and normal tissues in gynecologic cancer patients undergoing pelvic IMRT remain unclear. Investigators at Washington University reported the use of the following constraints: PTV (100% to receive 95% of the prescription dose), small bowel (<40% to receive ≥30 Gy), rectum (<40% to receive ≥40 Gy), and femoral heads (<40% to receive ≥30 Gy) (95). In the original series from the University of Chicago, <40% of the small bowel, rectum and bladder were constrained to receive ≥36, ≥40 and ≥40 Gy, respectively. Moreover, >95% of the PTV needed to receive >95% of the prescribed dose (96).
In recent years, detailed normal tissue complication probability (NTCP) studies have been performed in gynecologic cancer patients undergoing pelvic IMRT which shed light on the optimal dose–volume constraints for various normal tissues (Fig. 25.6) (97,98,99). In update of the combined University of Chicago/University of California San Diego experience, the small bowel volume receiving ≥45 Gy was constrained to 250 cc and the volume of pelvic bone marrow (defined as entire pelvic bones) receiving ≥10 and ≥20 Gy were constrained to receive ≤90% and ≤75%, respectively (100). Current attention is focused on the use of more sophisticated image-guided approaches in an effort to further optimize IMRT planning (101).
Preliminary clinical outcome studies in gynecologic cancer patients undergoing pelvic IMRT have been extremely promising, with lower rates of acute and chronic toxicities reported compared to conventional techniques (96,98,100,101,102,103). Recent outcome studies have also reported excellent tumor control rates in both cervical
(95,100) and uterine (104) cancers. Favorable outcomes have also been reported using adjuvant pelvic IMRT on the RTOG 0418 prospective clinical trial (105,106). An international cooperative group trial evaluating pelvic IMRT in cervical cancer is currently under development (107).
(95,100) and uterine (104) cancers. Favorable outcomes have also been reported using adjuvant pelvic IMRT on the RTOG 0418 prospective clinical trial (105,106). An international cooperative group trial evaluating pelvic IMRT in cervical cancer is currently under development (107).
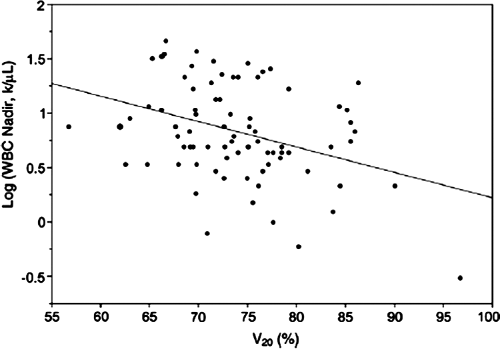 Figure 25.6. Plot of white blood cell count log (WBC nadir) versus bone marrow volume receiving 20 Gy or more (V20) in a cohort of cervical cancer patients treated with combined chemoradiotherapy, supporting the use of bone marrow V20 as a constraint in the optimization process. Pelvic bones were used as a surrogate for pelvic bone marrow in this analysis. Regression coefficient (b) = -0.021 k/mL/%, p = 0.002. Source: From Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010;78:912–919, with permission.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|