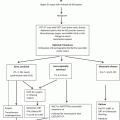
Fig. 7.1
The algorithm for treatment strategies of esophageal carcinoma
7.3 Epidemiology and Current Status of Esophageal Carcinoma in Japan
In Japan, the incidence rate of esophageal carcinoma has been increasing gradually in male, whereas it has been leveling off in female. The mortality has been leveling off in male, but been decreasing in female [2].
The percentage of males is higher with a male-female ratio of about 6:1. Most patients were in their 60s or 70s, accounting for about 68 % of all patients. The most frequent site of primary tumor is the middle thoracic esophagus (51.6 %). Squamous cell carcinoma is the predominant histologic type in Japan [2]. Esophageal carcinoma is also frequently associated with synchronous or metachronous multiple carcinoma.
Alcohol drinking and smoking are important risk factors for squamous cell carcinoma, serving as risk factors in more than 90 % of all cases of esophageal carcinoma in Japan. As for the risk factors for adenocarcinoma, Barrett’s epithelium derived from persistent inflammation of the lower esophagus due to gastroesophageal reflux disease (GERD) has been reported in western countries.
The estimated incidence rate in 2004 (crude incidence rate) was 24.4 persons per 100,000 population in male and 4.0 persons per 100,000 population in female [3]. According to a survey of the demographic trends conducted by the Ministry of Health, Labour and Welfare, there were 11.746 deaths from esophageal carcinoma in 2008 (crude mortality rate 9.3 persons per 100,000 population), which accounted for 3.4 % of all deaths from malignant neoplasms [3]. The age-adjusted mortality rate of esophageal carcinoma has been leveling off in men and decreasing in women [3].
7.4 Diagnosis of Esophageal Carcinoma
Clinical stage of esophageal carcinoma is determined by various diagnostic-imaging procedures in terms of the depth of tumor invasion and status of lymph node involvement and distant metastasis. Clinical staging is essential to decide therapeutic strategy for individual patients. Radical esophagectomy with lymph node dissection is one of the most invasive surgical procedures among various types of gastrointestinal surgery. The incidences of postoperative complications after radical esophagectomy and surgery-related mortality still remain higher than those for other procedures [4]. Multimodal approaches including chemoradiotherapy make the invasiveness of treatment much higher and complicated. It should also be noted that elderly patients are more likely to have various comorbidities including hypertension, diabetes mellitus, and hyperlipidemia. Therefore, it is desirable that the functions of vital organs meet certain criteria for implementation of the multimodal therapy.
From these reasons, several tests evaluating performance status, pulmonary function, cardiac function, hepatic function, renal function, glucose tolerance, and central nervous system function are required to decide therapeutic strategy for patients. However, application of therapy based on the patient’s general condition should follow comprehensive evaluation [5]. Patients should be informed of the therapeutic strategies based on the assessment of the clinical stage and their general condition.
7.5 Endoscopic Treatment
Endoscopic treatment includes the conventional endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), photodynamic therapy (PDT), argon plasma coagulation therapy, and electromagnetic coagulation therapy.
ESD enable us to perform en bloc resection of an extensive lesion using various types of knives [6].
Among lesions that do not exceed the mucosal layer (T1a), those remaining within the mucosal epithelium (EP) or the lamina propria mucosae (LPM) are extremely rarely associated with lymph node metastasis; therefore, endoscopic resection is considered as a sufficiently curative treatment for these lesions [7].
Mucosal resection covering 3/4 of the entire circumference is likely to be associated with postoperative stenosis. In cases of superficially spread lesions, deep infiltration may occur in several areas, necessitating careful diagnosis of the depth of invasion.
It is also difficult to accurately determine the depth of invasion of extensive lesions or fragmented specimens. Thus, tissue specimens obtained by en bloc resection are crucial. Handling and pathological evaluation of resected specimens are critical to decide the indication of additional treatments after endoscopic resection. Therefore, precise rules for handling resected specimens are described in the 2012 edition of guidelines [1].
Various complications, including bleeding, esophageal perforation, and serious stenosis, have been reported in association with endoscopic resection. There has been extensive discussion on the need for additional treatments after non-curative endoscopic treatment.
7.6 Surgical Treatment
Although there are various options for therapeutic strategy for esophageal cancer according to the location of the tumor, stage, and general condition of the patient, surgical treatment remains the mainstay of treatment. There are also various options depending on the institution as to the width of the resection margin, extent of lymph node dissection, the organ and route used for reconstruction, multimodal treatment including adjuvant therapy, and salvage surgery following definitive chemoradiation.
7.6.1 Surgery for Cervical Esophageal Carcinoma
The anatomical structure and physiological functions of the hypopharynx to the cervical esophagus are complicated. The surgical procedure should be determined carefully because the loss of vocal function by combined laryngectomy largely affects the postoperative QOL of the patient seriously.
7.6.2 Surgery for Thoracic Esophageal Carcinoma
Thoracic esophageal carcinoma is often associated with extensive lymph node involvements in the cervical, thoracic, and abdominal regions. Right thoracotomy with total extirpation of the thoracoabdominal esophagus and lymph node dissection in all the three regions (cervical, thoracic, and abdominal) is generally carried out in Japan [8, 9]. Intensive lymph node dissection along bilateral recurrent laryngeal nerves is essential and these procedures are the most demanding.
Three routes of reconstruction, i.e., antethoracic, retrosternal, and posterior mediastinal, are available. Although these routes have its own advantages and disadvantages, the posterior mediastinal route has been the most frequently employed recently. Stomach is the most common organ used for reconstruction.
Although thoracoscopy- or laparoscopy-assisted esophagectomy and mediastinoscopy- or laparoscopy-assisted transhiatal esophagectomy have been reported as promising surgical procedures, they are still under investigation, in view of the minimal invasiveness and oncological safety. It has been reported that thoracoscopic esophagectomy is comparable to conventional standard thoracotomic surgery in terms of the operating time, amount of blood loss, and number of dissected lymph nodes and is advantageous in terms of providing early recovery from postoperative pain and rapid restoration of vital capacity, as long as it is carried out at institutions with accumulated clinical experience [10, 11].
Although thoracic manipulations were predominantly carried out with the patient in the left lateral decubitus position previously, complete thoracoscopic procedures with the patient in the prone position have been introduced recently in Japan [12].
However, no definitive conclusions have been arrived yet as to the long-term outcomes of this form of minimally invasive esophagectomy as compared with those of conventional standard open esophagectomy with node dissection, and further investigation in randomized controlled trials is required.
7.6.3 Surgery for Carcinoma of the Esophagogastric Junction (Abdominal Esophageal Carcinoma)
The 10th edition of the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus defines the esophagogastric junction region as the region within 2 cm above and below the esophagogastric junction and esophagogastric junction carcinoma as carcinoma with its center located within this region [13]. In cases of esophagogastric junction carcinoma extending more to the esophageal side than to the gastric side (E, EG), right thoracotomy with dissection including the upper mediastinal lymph nodes and reconstruction using a gastric tube are performed in the same manner as for cases of thoracic esophageal carcinoma. In some cases, lower esophagectomy with proximal gastrectomy or lower esophagectomy with total gastrectomy via left thoracolaparotomy or serial left thoracoabdominal incisions may be carried out, considering that cervical or upper mediastinal lymph node dissection is of lesser significance. A transhiatal approach to the lower mediastinum without thoracotomy is also reported. In cases of esophagogastric junction carcinoma extending more to the gastric side than to the esophageal side (G, GE), metastasis to the mediastinal lymph nodes is less frequent; thus dissection of these lymph nodes is of lesser consequence. Therefore, these lymph nodes are classified as group 3 in the 10th edition of the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus.
7.6.4 Transhiatal Esophagectomy
In transhiatal esophagectomy, the thoracic esophagus is mobilized via the cervical and abdominal approaches without thoracotomy. This technique has been employed mainly in lower thoracic esophageal carcinoma or carcinoma of the esophagogastric junction in western world. RCT conducted in the Netherlands could not show survival benefit of transthoracic esophagectomy for adenocarcinoma on EG junction in comparison with transhiatal esophagectomy [14]. Now, optimal extent of lymph node dissection for carcinoma of the esophagogastric junction is controversial and under investigation.
Currently, the indication of transhiatal esophagectomy has become limited because of the spread of chemoradiotherapy and endoscopic submucosal dissection in Japan.
7.6.5 Perioperative Management and Clinical Path
In recent years, a clinical path for resection and reconstruction of the esophagus has been proposed by various institutions and been applied in clinical practice. However, there have been only limited data from large-scale clinical studies evaluating the usefulness of a clinical path for perioperative management.
Many institutions have introduced nutritional support teams (NST) for perioperative nutritional management of patients with esophageal carcinoma, facilitating early implementation of enteral nutrition [15]. In patients undergoing radical surgery for esophageal carcinoma, it has been considered that early enteral nutrition rather than central venous nutrition is desirable to maintain the postoperative immunity. An enteral feeding tube should be placed during surgery, and a liquid diet should be initiated by 1–3 days after surgery. As an element of perioperative management, steroid administration is useful and recommended in perioperative management [16]. Abstinence from smoking, respiratory physical therapy, and preoperative oral care are generally considered to be important for the prevention of postoperative complications.
7.6.6 Salvage Surgery
The 10th edition of the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus defines salvage surgery as surgery for residual or recurrent cancer after definitive (chemo)radiotherapy with 50 Gy or more as total irradiation dose [13]. The incidence of complications is higher in cases of salvage surgery than in patients treated by surgery alone or surgery combined with preoperative chemoradiotherapy (radiation dose less than 50 Gy). The reported in-hospital mortality after salvage surgery is 7–22 %, indicating that this type of surgery is associated with a higher surgical risk than usual surgery [17]. The high incidence of complications and high in-hospital mortality should be taken into account when considering the indications for salvage surgery.
Currently, no treatment other than salvage treatment including endoscopic resection is accepted as curative treatment for residual or recurrent tumor after definitive chemoradiation. However, salvage surgery must be undertaken only with the informed consent of the patients obtained after explaining the risks and long-term outcomes, and thus requires cautious consideration.
7.7 Neoadjuvant Therapy
This is the most significantly updated part in the 2012 edition of guidelines. A number of randomized controlled trials have been conducted in western countries addressing the possible beneficial effects of neoadjuvant chemotherapy on the survival rates of patients with esophageal carcinoma. According to the results of a meta-analysis of these randomized controlled trials, the effects of neoadjuvant chemotherapy on the survival of the patients varied and had been unclear [18]. Therefore, the 2007 edition of guidelines recommended the implementation of adjuvant chemotherapy particularly in patients with positive lymph node metastasis, on the basis of the results of the JCOG (Japan Clinical Oncology Group) 9204 study (1992–1997: postoperative adjuvant chemotherapy with cisplatin + 5-FU vs. surgery alone) [19]. The randomized controlled trial (JCOG9907 study) that compared neoadjuvant chemotherapy and postoperative chemotherapy with cisplatin + 5-FU in patients with resectable stage II or III thoracic esophageal carcinoma (2002 UICC classification) revealed a significant improvement in the overall survival in neoadjuvant group [20]. Based on this finding, neoadjuvant chemotherapy + radical surgery for resectable stage II or III thoracic esophageal carcinoma is recognized as a standard treatment in Japan.
On the other hand, neoadjuvant chemoradiotherapy is a mainstay in the multimodal treatment for esophageal carcinoma in western countries. According to a meta-analysis that addressed surgery preceded by neoadjuvant chemoradiotherapy vs. surgery alone, when the 3-year survival rate was estimated as an endpoint, neoadjuvant chemoradiotherapy (20–45 Gy) in patients with resectable esophageal carcinoma was associated with a significant increase in operation-related mortality within 90 postoperative days, but resulted in a decrease in the local recurrence rate and significant increase of the 3-year survival rate [21].
In meta-analyses carried out so far in the West, the patient population (histologic type, stage, etc.) and chemoradiotherapy protocols have not been consistent. The quality of surgery has been suggested to greatly influence the outcome. No randomized controlled trials of neoadjuvant chemoradiotherapy have been carried out to date in Japan, and thus at present, there is no satisfactory rationale for recommending this therapy as effective neoadjuvant treatment.
7.8 Postoperative Adjuvant Therapy
7.8.1 Postoperative Chemotherapy
A randomized controlled trial (JCOG9204 study) comparing surgery with and without postoperative chemotherapy (cisplatin + 5-FU, 2 courses) conducted in Japan demonstrated that postoperative chemotherapy resulted in a significant improve in the disease-free survival as compared to surgery alone. However, there was no significant difference in the overall survival [19]. Subgroup analysis from the JCOG9204 study demonstrated that the recurrence-preventive effect of 2 courses of cisplatin + 5-FU therapy administered postoperatively was observed only in patients with positive lymph node metastasis; therefore, in clinical practice, postoperative adjuvant chemotherapy has been recommended only after referring to the results of pathological examination after radical surgery. However, according to the results of the JCOG9907 study, implementation of neoadjuvant chemotherapy has been recognized as a standard treatment as describe above.
7.8.2 Postoperative Radiotherapy
The results of a randomized controlled trial of pre- and postoperative radiotherapy vs. postoperative radiotherapy alone carried out by the JCOG showed that the overall survival rate was significantly higher in postoperative radiotherapy alone group when the analysis was focused only on eligible patients who received treatment according to the protocol. Based on this finding, preventive postoperative irradiation was once in widely used in Japan. On the other hand, in randomized controlled trials in the West that compared surgery with and without postoperative irradiation (usual fractionation, 45–60 Gy), postoperative irradiation was associated with a decrease in the local recurrence in the irradiated area, but without a significant increase in the survival rate. Therefore, there is little evidence for recommending postoperative irradiation after curative resection as a standard treatment. At present, the significance of postoperative (chemo)radiotherapy is unclear. (Chemo)radiotherapy has been employed in clinical practice and also been reported to be effective, for cases of non-curative resection or postoperative local recurrence. Although there is insufficient evidence, some local therapy may be necessary for patients who have undergone non-curative resection and who have macroscopic residual tumor without distant metastasis. (Chemo)radiotherapy seems to be a useful treatment option for such patients.
7.9 Chemotherapy
Chemotherapy in the treatment of potentially resectable esophageal carcinoma is usually combined with surgery or radiotherapy in preoperative or postoperative setting. The application of chemotherapy alone is limited to patients with distant metastasis (M1b) or postoperative distant organ recurrence. Currently, 5-FU + cisplatin is the most commonly used regimen for esophageal squamous cell carcinoma in Japan. However, since there is no definitive evidence of prolongation of the survival period, this therapy is regarded as a palliative treatment.
7.9.1 Proven Effective Monotherapy Drugs
While 15–44 % of patients have been estimated to respond to monotherapy, cases of complete response (CR) are rare, and no monotherapy has been shown to have survival benefit [22]. At present, the most commonly used drugs are 5-FU and cisplatin. Basic studies have demonstrated that these two drugs are effective when used as monotherapy and exert a synergistic effect when combined with some other drugs and a sensitizing effect when combined with radiotherapy. A few reports of these drugs yielding good results when used in combination in the clinical setting have also been published. These are the reasons for the wide use of these two drugs.
7.9.2 Combination Therapy
Although various combination therapies using cisplatin have been employed since this drug was introduced clinically, the currently most commonly used combination regimen is 5-FU + cisplatin [23]. Recently, regimens containing paclitaxel, irinotecan, or gemcitabine have been tried in the West [24], and regimens using nedaplatin or docetaxel have been tried in Japan; no large-scale phase III trials of these regimens have been carried out. Thus, the survival benefit of these regimens over the standard combination of 5-FU + cisplatin has yet to be demonstrated. Currently in Japan, the combination of 5-FU + cisplatin is commonly used as the first-line treatment, following by docetaxel as a second-line treatment. In any event, the effect of the use of chemotherapy alone, regardless of whether it is combination therapy or monotherapy, is limited, and chemotherapy not combined with other treatment modalities is applied only to patients with unresectable metastatic lesions.
Cisplatin, a chemotherapeutic drug that is in wide use, is classified as a highly pro-emetic drug. Guidelines for appropriate use of antiemetic drugs recommend the triple-drug combination of a 5-HT3 receptor antagonist, corticosteroid, and aprepitant to prevent emesis while using cisplatin. For other drugs, it is necessary to check the risk of emesis against guidelines for appropriate use of antiemetic drugs and to take appropriate prophylactic measures.
7.10 Radiotherapy
Previously, radiotherapy was primarily used for patients who were not suitable candidates for curative surgery or endoscopic resection. However, in recent years, radiotherapy (particularly, chemoradiotherapy) has been widely used for both superficial carcinoma and locally advanced carcinoma, as radical treatment.
Details of the standard radiotherapy used for esophageal carcinoma are described in the Radiotherapy Planning Guidelines 2008 (ed. by Japanese College of Radiology, Japanese Society for Therapeutic Radiology and Oncology, and Japan Radiological Society) [25].
As compared to radiation alone, concurrent chemoradiotherapy significantly increases the survival rate, although radiotherapy administered sequentially after induction chemotherapy does not [26]. Concurrent chemoradiotherapy is indicated for medically fit patients with T1-4N0-3M0 carcinoma (UICC-TNM classification, 2009 edition) and those with locally advanced carcinoma up to metastasis to the supraclavicular lymph nodes (M1) [27]. However, the risk of serious complications such as fistula formation is high in cases of unresectable locally advanced carcinoma (T4).
Because prolongation of the duration of irradiation decreases the local control rate of radiation monotherapy, it is important to complete irradiation using a radical dose (66–68.4 Gy) within 7 weeks. In radical concurrent chemoradiotherapy, use of at least 50 Gy/25 times/5 weeks by the usual fractionation protocol is necessary. The standard radiation dose for concurrent chemoradiotherapy in the USA is 50.4 Gy/28 times [28]. In contrast, in Japan, the standard radiation dose is 60 Gy/30 times/6–8 weeks for concurrent chemoradiotherapy, and its safety has already been demonstrated [29].
A randomized controlled trial carried out in Japan revealed that combined use of external radiation and intraluminal brachytherapy is effective for patients with T1-2 esophageal carcinoma, a relatively early stage of the disease [30]. However, recently chemoradiotherapy is used commonly, and the available evidence is not sufficient to recommend the addition of intraluminal brachytherapy to chemoradiotherapy.
7.11 Chemoradiotherapy
Randomized controlled trials have demonstrated that chemoradiotherapy has a significantly higher survival rate in comparison to radiation alone in patients with esophageal carcinoma; therefore, this therapeutic modality is regarded as the standard therapy for patients with esophageal carcinoma who are not suitable for surgical treatment [31]. Furthermore, definitive chemoradiotherapy is also indicated for resectable T1-3N0-3M0 cases (UICC-TNM classification, 2009 edition), unresectable T4N0-3M0 cases, and cases with metastasis to lymph nodes other than the regional lymph nodes (M1). There are several reports that have demonstrated the absence of any significant difference in the overall survival and disease-free survival between patients with resectable lesions treated by definitive chemoradiotherapy or by surgery alone [32]. However, in Japan, neoadjuvant chemotherapy followed by surgery is expected to be superior to chemoradiotherapy in patients with stage IB-III disease (UICC-TNM classification, 2009 edition), while equivalence of chemoradiotherapy and surgery is expected in patients with stage IA disease (T1N0M0, UICC-TNM classification, 2009 edition) [33, 20]. Although the chemo-intensity, irradiation doses, and treatment schedules vary among different clinical trials, the most common protocol employed is combined chemotherapy with 5-FU plus cisplatin and concurrent radiotherapy at a total dose of 50–60 Gy. It is necessary to recognize that any reported treatment results are based on the assumption of adequate chemotherapy and radiotherapy.
7.11.1 An Optimal Dose of Irradiation and Regimen of Chemotherapy
A randomized controlled study (RTOG9405/INT0123) carried out by the RTOG that compared chemoradiotherapy using standard-dose (50.4 Gy) and high-dose (64.8 Gy) radiation in patients with T1-4N0-1M0 esophageal carcinoma (corresponding to UICC-TNM classification, 2002 edition) revealed no superiority of high-dose radiation over standard-dose radiation in terms of the median survival time, the 2-year survival rate, and the local control rate and concluded that the standard radiation dose for chemoradiotherapy using a combination of 5-FU plus cisplatin should be 50.4 Gy (1.8 Gy × 28 times) as described above [28]. On the other hand, a radiation dose of 60 Gy has been used commonly in Japan. Although the standard radiation dose has not yet been established in Japan, change to 1.8 Gy/fraction × 28 times (total dose of 50.4 Gy) is now under clinical investigation.
The standard chemotherapy regimen for concurrent chemoradiation is 5-FU + cisplatin. In the RTOG9405/INT0123 study, a course of 4-day continuous intravenous infusion of 5-FU at 1,000 mg/m2/day plus intravenous cisplatin at 75 mg/m2 on day 1 was repeated every 4 weeks up to a total of 4 courses (concurrent radiation was used in the initial 2 courses) [28]. In Japan, although use of the 5-FU + cisplatin regimen is variable, a phase II clinical study (JCOG9708) of chemoradiotherapy (5-FU + cisplatin + irradiation of 60 Gy) for cases of stage I esophageal carcinoma (T1N0M0, UICC-TNM classification, 1997 edition [*corresponding to stage IA: T1N0M0 in the 2009 edition]) conducted by JCOG used 2 courses of 4-day continuous intravenous drip infusion of 5-FU at 700 mg/m2/day plus intravenous drip infusion of cisplatin at 70 mg/m2 on day 1 repeated every 4 weeks. In the JCOG9708 study, the complete response rate was 87.5 %, the 4-year survival rate was 80.5 %, and the 4-year progression-free survival rate was 68 %, suggesting equivalent results to those of surgery [33]. Currently, a phase III clinical study (JCOG0502) comparing definitive chemoradiotherapy with surgery alone is under investigation. In another phase II JCOG study (JCOG9906) of chemoradiotherapy (5-FU + cisplatin + irradiation of 60 Gy) performed in cases of resectable stage II–III esophageal carcinoma, a course of 5-day continuous intravenous infusion of 5-FU at 400 mg/m2/day for 2 weeks plus intravenous cisplatin at 40 mg/m2 on days 1 and 8 was repeated every 5 weeks for a total of 4 courses (the initial 2 courses were combined with concurrent irradiation) [34]. On the other hand, the introduction of chemotherapy according to the RTOG regimen is now under investigation in Japan.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree