Fig. 1
Classification of risk groups for survival of OPSCC using RPA analysis of 2 RTOG trials by Ang et al. (2010)
In 2013, Rietbergen et al. conducted a study, based on an unselected, consecutive cohort of 723 patients with OPSCC. This study also included patients with stage I/II disease and patients with moderate to severe comorbidity (using the ACE-27 score) (Kallogjeri et al. 2012; Kaplan and Feinstein 1974). HPV status was determined by p16-immunohistochemistry followed by an HPV DNA test on the p16-positive cases. Using this patient group, the prognostic model of the RTOG 0129 study was confirmed (Rietbergen et al. 2013); the 3-year survival rates were similar to those described previously (Ang et al. 2010). However, when analyzing this cohort using the RTOG 0129 prognostic model, the Harrell’s C-index was suboptimal. Therefore, an adapted recursive partitioning model was developed, based on this consecutive patient cohort, also including patients with stage I/II disease and patients with moderate to severe comorbidity. This new model confirmed that the major prognostic factor for patients with OPSCC is HPV status. However, comorbidity (instead of smoking) was the most important prognostic factor in HPV-positive patients and the second most important factor in HPV-negative patients after nodal stage (Fig. 2). In HPV-negative patients, nodal stage remained the most important prognostic factor. In HPV-positive patients, nodal stage did not influence the prognosis. The observation that HPV-positive patients have a good prognosis regardless of nodal stage has already been described in several studies (Chaturvedi et al. 2011; Shaw and Robinson 2011; Ang et al. 2010). Interestingly enough, smoking was not a prognostic factor in the prognostic model of Rietbergen et al. Smoking was one of the prognostic determinants of overall survival in the univariate and multivariable analyses. However, in the recursive partitioning analysis, comorbidity was a stronger prognostic factor than smoking. A likely explanation for this observation was that most of the patients in the cohort with a moderate to severe comorbidity were also heavy smokers (83.3 %), and died of smoking-related causes such as cardiovascular disease and lung, esophageal and head and neck cancers. In addition, most of the patients in the cohort smoked more than 10 PY (87.1 %), which is a very high percentage in comparison with other studies (Ang et al. 2010; O’Sullivan et al. 2013).
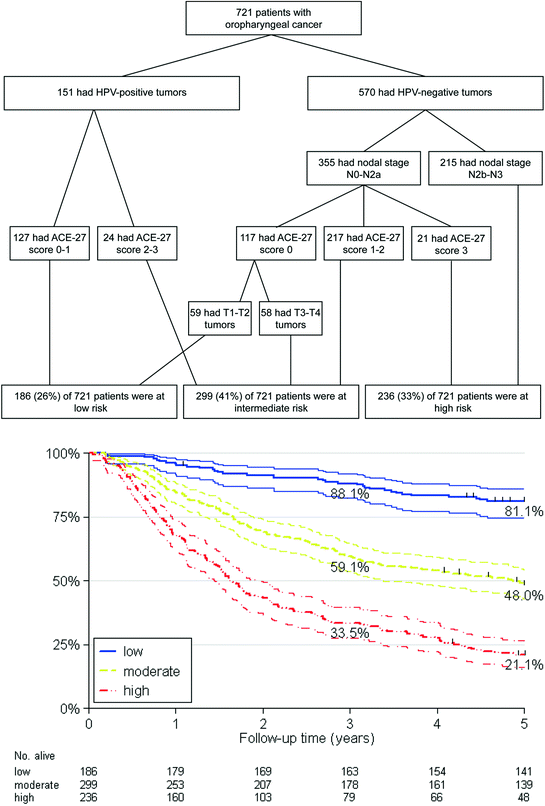

Fig. 2
Classification of risk groups for survival of OPSCC using RPA anlysis of a Netherlands cohort by Rietbergen et al. (2013b)
In 2015, the prognostic model proposed by Rietbergen et al. (2015) was validated with an independent series of patients. Whereas the RTOG 0129 prognostic model focuses on the so-called ‘trial population’ (i.e., patients with stage III/IV OPSCC having a good ECOG performance score), this new prognostic model seems to be applicable to the entire population of patients presenting with OPSCC. Moreover, this model might be more suitable for a patient population with a high percentage of heavy smokers, as is the case in most European countries. Comorbidity, instead of tobacco smoking, might be a more informative prognostic factor in those populations.
One of the other remarkable findings in the study of Rietbergen et al. was the fact that survival of patients with p16-positive but HPV DNA-negative OPSCC (16.4 % of p16 positive patients) was significantly different compared to patients with ‘truly’ HPV-positive OPSCC. The survival curve of this ‘discordant’ group almost converged the survival curve of patients with an HPV-negative OPSCC. In 2011, Perrone et al. reported similar results; patients in the p16-positive but HPV DNA-negative subgroup showed the same overall survival curves as HPV-negative patients (Perrone et al. 2011). This finding might be important for the inclusion of patients in de-intensification trials. Currently, de-intensification trials are being conducted for which eligibility for randomization only involves positivity on p16-immunohistochemistry. However, this causes the risk to enroll patients with HPV DNA-negative tumors.
Therefore, we encourage to incorporate HPV status into the next (8th) edition of the TNM AJCC/UICC classification. Moreover, we stress the importance of performing reliable HPV DNA testing besides p16-immunohistochemistry to detect a true HPV-related OPSCC.
3 De-intensification of Treatment
As patients with HPV-positive OPSCC have a favorable prognosis, an opportunity now exists to investigate less intense treatment strategies for these patients. These treatment strategies should not compromise survival outcomes but lower the risk of potentially debilitating late effects of treatment. For the most part, patients with HPV-positive OPSCC are younger and generally have a better health status compared to patients with HPV-negative OPSCC. Thus, low treatment-related toxicities and a high level of quality of life after treatment are important considerations in the clinical management of these patients.
In 2010, Ang et al. already suggested, on the basis of their data, that future clinical trials should be designed specifically for patients with HPV-positive OPSCC. Their analysis did not show a significant difference in overall survival between a concomitant-boost accelerated-fractionation regimen of radiotherapy and a standard-fractionation regimen, combined with concurrent, high-dose cisplatin in patients with HPV-positive OPSCC. Therefore, they suggested that either regimen could serve as the comparison for a new therapy being investigated.
Currently, several de-escalation trials are running for patients with HPV-positive OPSCC. The DeESCALATE-HPV trial is a phase III trial that compares radiotherapy plus cetuximab versus chemoradiotherapy in patients with HPV-positive OPSCC. The purpose of this study is to evaluate the application of cetuximab instead of cisplatin, a less toxic alternative for concurrent chemoradiation. A second trial that aims to assess a potential for cetuximab instead of cisplatin, is the (recently closed) RTOG 1016 trial.
Another de-escalation trial is the recently closed ECOG-E1308 phase II trial, for patients with stage III/IV HPV-positive OPSCC. This trial tested whether induction chemotherapy (combination of paclitaxel, cisplatin, and cetuximab followed by concurrent cetuximab and radiotherapy) may allow for safe reduction in radiotherapy dose to the primary site and involved neck nodes. Patients with complete response to induction had modification of the prescribed radiation therapy dose from 69.3 Gy (given for incomplete response) to 54 Gy. Eligibility for randomization in this trial also included HPV16 in situ hybridization besides p16-immunohistochemistry.
The ability to estimate survival probability of OPSCC patients before any type of treatment would be very valuable for decision making, especially for patients who might be enrolled in treatment de-escalation trials. The two prognostic models described above might be used to stratify patients for de-intensification therapy. However, when considering treatment selection, it may also be useful to stratify patients based on their risk of recurrence. Despite the known favorable locoregional and survival outcome of HPV-positive compared with HPV-negative OPSCC, the recent literature shows that distant metastases (DM) rates are the same for both (Ang et al. 2010; O’Sullivan et al. 2012). In addition, DM in HPV-positive patients may occur in unexpected sites and after longer intervals (Huang et al. 2012). DM seems to be the leading cause of death in HPV-positive patients. O’Sullivan et al. (2013) recently demonstrated that HPV-positive patients with nodal stage N2c have a reduced distant control when treated with radiotherapy alone and seem less suited for deintensification strategies that omit chemotherapy. They suggest that deintensification that withholds or reduces chemotherapy intensity should be considered cautiously and might be best deployed in subgroups least likely to develop DM (i.e., T1-3 N0-2c patients).
4 Refinement of Prognostic Models by Other Biomarkers
Besides the main prognostic factors for survival in patients with HPV-positive OPSCC (i.e., smoking, comorbidity, and a high nodal stage), other predictors of survival have recently been suggested. Recent investigations undertaken to improve the staging system for HPV-positive tumors suggest that new biomarkers to complement known risk factors are needed to improve prognostic accuracy (Huang et al. 2012; Rios et al. 2014).
Murphy et al. recently described the relationship between tumor-specific growth rate (TSGR) and oropharyngeal cancer (OPC) outcomes in the HPV-positive patient. TSGR was defined as the primary tumor volume differences between a diagnostic and secondary scan separated >7 days without interval treatment (in percent volume growth/day) (Murphy et al. 2015). This was derived from primary tumor volume doubling time for 85 OPC patients with known p16 status and smoking pack-years managed with (chemo)radiation. TSGR was incorporated into RTOG 0129 risk grouping (0129RG) to assess whether TSGR could improve prognostic accuracy. Incorporation of this radio biomarker into risk stratification with 0129RG improved the predictive quality of the risk groups. It suggests the potential ability of TSGR to improve patient selection for treatment intensification.
Another potential biomarker could be tumor infiltrating lymphocytes (TIL) levels. In 2014, Ward et al. (2014) showed that TIL levels predict for survival in OPSCC patients. In their study, survival in patients with HPV positive, TILlow tumors, was not significantly different than in those with HPV-negative disease. A prognostic model based on low TIL levels, heavy smoking, and late T stage allowed the identification of a group of HPV-positive patients with poor survival.
The prognostic impact of EGFR overexpression remains uncertain. EGFR is abnormally activated in approximately 80 % of head and neck cancers (Thomas et al. 2005). EGFR expression has been associated with prognosis in head and neck cancer, particularly in patients treated with radiotherapy, although some studies have found no relationship between EGFR and treatment response or outcome (Thomas et al. 2005; Putti et al. 2002; Chang et al. 2008; Kong et al. 2009; Fischer et al. 2008).
Hong et al. (2010) examined the prognostic significance of EGFR expression in relation to HPV status in 270 OPSCCs. Their data showed that EGFR and HPV are independent prognostic markers in OPC, although the effect of EGFR was more convincing for locoregional control than for survival. They suggested that use of EGFR in combination with HPV status gives additional prognostic information particularly in terms of locoregional control.
Vainshtein et al. (2014) investigated EGFR overexpression in 184 HPV-positive and 14 HPV-negative patients. EGFR overexpression was related to HPV-negative status and was univariately associated with locoregional (LR) recurrence in the overall population, but was neither retained in the multivariate model after adjustment for HPV status, nor associated with LR recurrence in HPV-positive patients. In HPV-positive patients, only T4- and N3-stages were significant predictors of LR recurrence on multivariate analysis.
In 2015, another potential biomarker was investigated that might influence survival in HPV-positive OPSCC patients; CD98. CD98 has been described as being a novel enrichment marker for cancer stem cells (CSCs) (Martens-de Kemp et al. 2013). CSCs represent a small subpopulation of tumor cells that maintain tumor growth by fuelling the expansion of the malignant cell population infinitely (Bao et al. 2006). CSCs can be distinguished from the bulk of the tumor based on differential expression of protein markers on the cell membrane. Previous studies suggest that treatment failure in head and neck cancer patients might be the consequence of therapy resistance of CSCs (de Jong et al. 2010). Rietbergen et al. (2014) showed that overall survival and progression-free survival was markedly better for HPV-positive patients with a CD98low OPSCC compared to HPV-positive patients with a CD98high OPSCC. Consequently, CD98-expression could be used as an additional prognostic marker for selection of HPV-positive patients in clinical trials. Although a challenging and attractive idea, the use of CD98 as a prognostic marker should first be preceded by a thorough validation phase in prospective clinical trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree