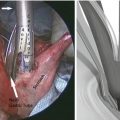
Fig. 4.1
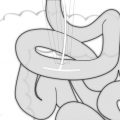
(a) The lymphatics of the esophagus are distributed in the form of a submucosal and a paraesophageal plexus that can both drain directly into the periesophageal lymph nodes. (b) Longitudinal spread of tumor to involve submucosal lymphatic plexus (From Elsevier; Raja et al. [116] with permission)
An esophageal resection can be suboptimal due because of an involved circumferential margin. The definition of circumferential resection margin (CRM) involvement remains controversial. The College of American Pathologists (CAP) and the Royal College of Pathologists (RCP) use different definitions for CRM involvement. Microscopic tumor involvement (R1 resection) is defined by CAP as tumor found at the cut circumferential resection margin, while it is defined by RCP as any tumor within 1 mm of the circumferential resection plane. Recently, a systematic review was published of 14 studies involving 2,433 patients. Rates of CRM involvement were 15.3 and 36.5 % according to the CAP and RCP criteria, respectively. It was shown that CRM involvement is an important predictor of poor prognosis and that the CAP criteria had a greater (negative) prognostic power than the RCP criteria [48]. It can be difficult and time-consuming to identify a positive circumferential resection margin in a large T3 tumor, and it has been suggested that this should preferably be done in accordance with the CAP criteria (tumor is found at the inked lateral margin of resection) [49]. There has been a significant decrease in CRM involvement especially with the introduction of neoadjuvant chemoradiotherapy [17, 50]. After neoadjuvant chemotherapy CRM involvement still has prognostic importance [51].
Lymphadenectomy
As esophageal cancer readily spreads longitudinally in the submucosal lymphatics, early dissemination to lymph nodes in the chest and abdomen may be involved in cancer of all parts of the esophagus. And even skip metastases, defined as positive distant lymph nodes in combination with negative regional lymph nodes, are encountered relatively frequently [52]. Lymphatic dissemination occurs not only in a chaotic pattern but also at an early stage. Some 30 % of the T1b tumors (with infiltration limited to the submucosa) already have positive lymph nodes involved [53]. Ideally, a complete resection of all locoregional nodes draining the esophagus should include the two or three fields (see above) in addition to the easily accessible periesophageal and perigastric lymph nodes (Fig. 4.2). In a survey among surgeons around the world, the technically challenging three-field lymphadenectomy was performed routinely by only 12 % of the responders [54]. A SEER analysis showed that the median number of total lymph nodes resected in over 5,600 esophagectomies was only eight nodes [55]. Lymphadenectomy can be performed safely during minimally invasive surgery, and it has been shown that minimally invasive and robotic esophagectomy have similar lymph node retrieval compared to open techniques [56–58].
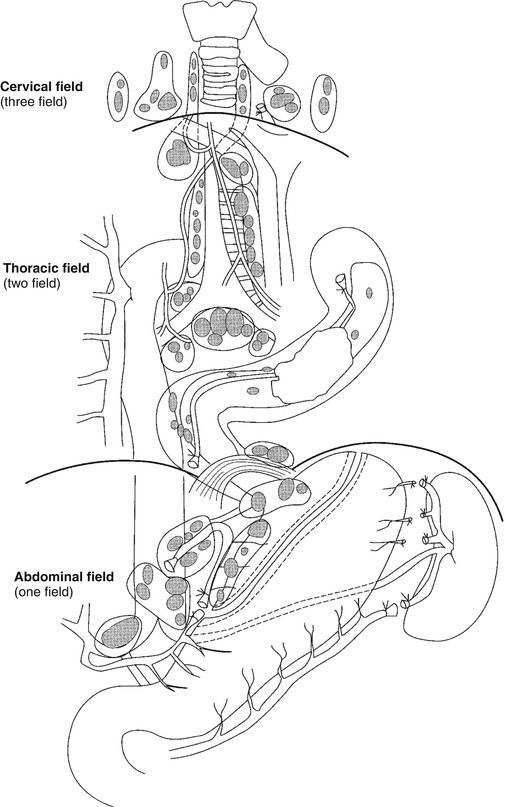

Fig. 4.2
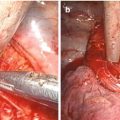
Extent of resection and fields of lymph node dissection routinely carried out for cancer of the esophagus (From Griffin and Raimes [117] with permission)
For staging purposes it is clear that an extended lymphadenectomy is superior to a limited dissection. It has, therefore, been suggested by the 7th edition of the TNM staging system that for staging purposes, the total number of resected and identified lymph nodes should be at least 15 nodes. The therapeutic impact of an extended lymphadenectomy is still a matter of debate in esophageal cancer surgery [59]. Some authors state that surgery has reached its limit, while others believe that the course of the disease can be influenced positively by aggressive surgery with an extended lymphadenectomy. One of the hypotheses supporting the benefits of extended lymphadenectomy is the clearance of micrometastases that can be present in up to 50 % of histology-negative nodes. This hypothesis is supported by the correlation of micrometastases in routine lymph node-negative patients with a poor outcome [60, 61].
More skeptical authors believe that the therapeutic impact of an increased lymph node harvest per se is limited and it is probably not the type of operation performed that makes a difference but rather the stage of the disease at the time of operation [56]. According to this view, lymph node metastases are markers of systemic disease and removal of the primary lesion alone will yield the same survival [62]. The spurious effect of extended lymphadenectomy might then be caused by stage migration which occurs if positive nodes in the extended field change N-stage. This results in the so-called Will Rogers phenomenon or stage purification and leads to unreliable stage-by-stage comparisons of survival. For that reason some authors prefer to use the lymph node ratio (i.e., the number of positive nodes over the number of removed nodes) rather than the absolute number of positive nodes [63, 64].
Several prospective trials have been performed comparing survival after esophagectomy with or without extended lymphadenectomy. In the largest RCT (HIVEX trial), comparing limited transhiatal esophagectomy and extended transthoracic esophagectomy with two-field lymphadenectomy, 5-year survival was not significantly different [65, 66]. The survival benefit of an extended lymphadenectomy by a transthoracic approach was limited to a subgroup of patients with low burden of nodal disease (1–8 nodes positive on pathological examination of the resection specimen). The identification of this group makes the pretreatment staging very challenging. Unfortunately, unlike in breast cancer, the sentinel node concept has not become popular in esophageal surgery [29, 31]. Several studies have confirmed the higher morbidity after thoracotomy than after transhiatal approach: more pulmonary complications, more recurrent nerve injuries, and higher early mortality [67–69].
Meta-analysis of the available literature data did not show differences in survival between transhiatal and transthoracic operations. Other studies compared fields of dissection, for example, the single-center studies by Lerut et al. [70] and Altorki et al. [71] that suggested a potential survival benefit for three-field lymphadenectomy.
Finally, there are studies that investigated the absolute number of nodes dissected. This has led to different recommendations regarding the optimal extent of lymphadenectomy ranging from 16 to 30 nodes. In a population of 4,627 patients in the Worldwide Esophageal Cancer Collaboration (WECC), extent of lymphadenectomy was not associated with increased survival for patients with extremes of esophageal cancer (TisN0M0 and 7 or more nodes positive) and those with well-differentiated pN0 cancer [72]. For all other cancers, 5-year survival improved with increasing extent of lymphadenectomy. Based on these WECC data, a stage-dependent extent of lymphadenectomy was recommended. This is comparable to the findings of the HIVEX trial that showed a better survival after a transthoracic approach in the subgroup of patients with 1–8 nodes positive [66]. Rizk et al. identified 18 nodes resected as the minimum necessary for accurate staging and for eliminating an effect of lymphadenectomy on survival [73]. In the study by Altorki et al., effect of lymphadenectomy on survival was lost after 25 nodes for early stage and after 16 nodes in stage III and IV cancers [71]. Peyre et al. investigated an international database of 2,303 esophagectomies in which survival was maximized with 23 nodes resected [74].
Nowadays, multimodality treatment of esophageal cancer has been widely accepted. As neoadjuvant chemoradiotherapy (CRT) is known to “sterilize” nodes, it is unclear whether the recommendations for number of lymph nodes from the surgery-alone era still stand. Extended lymphadenectomy seems to be beneficial, particularly in patients who are not downstaged regarding pathological tumor depth (ypT) and those with persistent nodal metastases (ypN+) [75, 76]. The effect of lymphadenectomy is influenced by tumor response after CRT, and the survival benefit is stronger in patients without a complete pathological response (non-pCR) compared to those with pCR [77].
Morbidity: Prevention of Complications
The typical esophageal cancer patient suffers from several comorbidities including obesity (especially in adenocarcinoma) and cardiopulmonary diseases (in both squamous and adenocarcinoma) that put the patient at increased risk for postoperative complications. Serious intraoperative and postoperative complications can occur with minimally invasive as well as open techniques, also depending on the need of a thoracic phase of the operation. Overall, complication rates are reported in over 50 % of esophagectomy series, with incidence varying between 17 and 74 % [78, 79]. Postoperative complications have been directly linked to a variety of other outcome parameters including mortality, readmission rate, early cancer recurrence, survival, length of hospital stay, costs and resource utilization, and quality of life [80–83]. The most important issues in the management of perioperative complications are prevention and early detection. However, a clear understanding of the relationships between complications, their recognition, management, and how they influence subsequent mortality is hampered by the lack of standardized definitions [84, 85]. Finally, early detection and proper management of postoperative complications is of crucial importance. It has been shown repeatedly that the so-called failure to rescue largely explains the difference in mortality rates between low-volume and high-volume hospitals for complicated surgery including esophagectomy [86].
The exact role for minimally invasive techniques is still not fully clear. The increased magnification offered by thoracoscopy might decrease complications, but lack of tactile control is probably a contributory factor to the increase of intraoperative injuries. It is unlikely that minimally invasive methods will reduce mortality rates since in experienced centers death after open esophagectomy is already a rare event. Minimally invasive esophagectomy (MIE) might be proven superior for other endpoints such as blood loss, duration of ICU or hospital stay, need for analgesics, and pulmonary function. The best available evidence comes from a recently published RCT (TIME trial) showing that MIE is accompanied by less pulmonary complications [87]. This trial has been criticized because of the lack of a clear definition of “pulmonary complications” as the primary endpoint [88]. Moreover, an unexplained increase of recurrent nerve injuries was present in the open group.
Respiratory Complications
Respiratory failure is a major problem after esophagectomy. Several studies have reported that about half of the inhospital deaths after esophagectomy is due to pneumonia, which is the most frequent general complication after surgery [89]. Preventive measures include preoperative respiratory training, cessation of smoking, and continuous postoperative pain control by epidural analgesia in order to avoid restrictive respiration and insufficient coughing. Micro-aspiration as a consequence of impaired swallowing coordination because of a cervical anastomosis also plays a role in the pathophysiology of bronchopneumonias. Another reason for postoperative respiratory impairment is a large pleural effusion, which should be drained if provoking extended atelectasis. Avoiding the need for a combined thoracotomy and laparotomy may potentially reduce postoperative pain, ventilator dependence, and cardiopulmonary complications [90]. In a study comparing thoracoscopic resection with a historical cohort, the overall incidence of pulmonary complications was reduced from 33 to 20 % [91]. Probably cardiopulmonary complications do not depend on the incision size only. The benefit of smaller port sites that are needed during minimally invasive surgery may be offset by the lengthened time of operation and single-lung ventilation. The use of a prone position also plays a role but will be discussed elsewhere.
Recurrent Laryngeal Nerve Injury
More recurrent laryngeal nerve injuries when using thoracoscopy have been reported, which might be attributed to the use of diathermia. Others claim that the use of minimally invasive techniques has lowered the incidence of hoarseness because of the magnified view [87].
Anastomotic Leakage
Lack of standardization of definitions is a problem when reporting on complications. In a recent meta-analysis, anastomotic leakage was reported in most of the publications, but it was defined in only a minority with 22 differing definitions [84]. Early disruption of the esophagogastric anastomosis is the result of a technical problem and immediate reexploration is frequently indicated for correction. Many different suturing and (semi-)mechanical techniques have been described. The semimechanical side-by-side technique claims a lower leakage rate compared to a hand-sewn anastomosis, but has not been tested in a randomized trial [92, 93]. Leakage is more frequent in the neck than in the chest, but the associated mortality might be lower, especially after a transhiatal approach [94]. If a transmural necrosis of the gastric conduit is suspected, this can be diagnosed by endoscopy and when present is also an indication for surgery with formation of a cervical esophagostomy, resection of the gastric tube, and placement of a feeding jejunostomy. After rehabilitation of the patient, a colonic interposition can be performed at a secondary stage. Late disruptions become manifest generally between postoperative day 5 and 10 and are most frequently due to ischemia. They can be managed nonoperatively in most cases with aggressive drainage using radiologically guided drains or endoluminal vacuum therapy [95]. Self-expandable stents can be inserted in these situations but can have the disadvantage of migration or further necrosis due to tissue compression ultimately leading to, e.g., neoesophago-tracheal fistula formation.
Chylothorax
The incidence of accidental thoracic duct leakage can be diminished by intraoperative identification and ligation of the duct. Reported incidence of chylothorax varies between 3 and 10 % and is seen more often in patients who undergo transthoracic esophagectomy and in patients who have more positive nodes. Patients with chyle leakage have more pulmonary complications. Conservative therapy (initial parenteral feeding and subsequent enteral diet with medium-chain triglycerides (MCT)) is often successful, but operative therapy should be seriously considered in patients with a persistently high daily output of more than 2 L after 2 days of optimal conservative therapy [96].
Cardiac Arrhythmias
Cardiac arrhythmias are not uncommon in the postoperative phase. Atrial fibrillation (AF) is seen in 15–20 % of patients and requires further investigation because it can be an early manifestation of, e.g., mediastinitis due to intrathoracic anastomotic leakage. AF can also be associated with hypervolemia, preexistent pulmonary or cardiac disease, and dilation of the gastric conduit.
Mortality and Quality Control
Definitions
There is an increasing interest in comparing institutional performance. For surgical procedures postoperative mortality rate is generally used, because it is a relatively objective measure and reflects the summation of the most severe postoperative complications. Currently it is unclear which definition of postoperative mortality best reflects surgical quality of care. The 30-day operative mortality (30DM) and the inhospital mortality (IHM) after esophageal resection are well documented and vary from 4 % for specialized centers to > 10 % for nationwide registries [97]. Few studies report on mortality beyond 30 days. Damhuis et al. however showed in the Dutch Cancer Registry that 43 % of inhospital deaths after surgery for esophageal cancer occurred 30 days or more after the operation [98]. Therefore, 90-day mortality (90DM) might be preferred as a performance indicator. Using a longer time period after the operation for defining postoperative mortality may thus provide a better definition of quality of surgery [99]. Extending the mortality period beyond 30 days and beyond inhospital stay has the advantage that patients who die because of surgery-related complications outside the hospital are included as well.
Not only short-term outcomes but also long-term survival should be part of the benchmark as both aspects are relevant for comparing surgical performance. Both surgery-related deaths and cancer recurrence-related deaths are reflections of surgical quality of care. Less radical surgical resections will generally result in lower postoperative morbidity and mortality but will generally give less favorable oncological outcomes.
Case Mix Correction
Even after agreement on a uniform definition of postoperative mortality, direct comparison of crude mortality rates between hospitals can be misleading as they do not take into account the case-mix difference, i.e., the differences in physiological condition and tumor stages of patients. Sophisticated models have been developed for prediction of 30DM and IHM [8, 14, 67, 100–104] after esophageal surgery, but models for 90DM have been mostly based on large multi-institutional databases with only few parameters available [105].
Outcome-Volume Relationship and Registration
Over the past decades, better long-term survival results have been presented, evolving from 18 % 5-year survival in the era from 1980 to 1990 to 48 % in the most recently published RCT (Table 4.1) [17, 65, 99, 106, 107]. It is suggested that many factors are responsible for this positive effect, including large hospital volume, early tumor detection, improved patient selection based on novel staging modalities, increased use of neoadjuvant therapy, better surgical and anesthesiological techniques, and improved standardized perioperative clinical pathways [18, 108]. In many countries around the world, it has been decided that high-risk surgical procedures such as esophagectomy should be restricted to facilities with a yearly minimum volume [109, 110]. It has been demonstrated that the incidence of postoperative complications is similar across hospitals but that the associated mortality rates are lowest in high-volume centers, which generally show a lower “failure to rescue” [86, 111]. Centralization is currently implemented widely. Also auditing has been implemented as a way of improvement of care. Of course this results in an additional registration burden for the surgeon, but comparing individual or institutional results with the benchmark has proven valuable in other types of cancer surgery, such as for rectal cancer [112, 113]. For esophageal cancer, variables of interest are, for example, hospital mortality, radicality (R-status), extent of lymph node dissection, length of hospital stay, application of neoadjuvant therapy, availability of PET-CT, and the presence of a well-structured MDT. The quality indicators can be divided in structural, process, and outcome measures, respectively (Table 4.2) [114]. Heterogeneity and lack of standardized definitions of the outcome of interest are a problem here as well. In a review of esophagectomy outcomes from 164 NSQIP (National Surgical Quality Improvement Project) hospitals, it was demonstrated that even following case mix adjustment, results between centers varied by 161 % for 30-day mortality and 84 % for serious morbidity [67].
Table 4.1
Several studies over previous decades showing improved long-term survival after esophageal resection
Study | Randomization | Survival |
|---|---|---|
Muller et al., 1990 [106] | N/A | 5-year survival 10 % |
Walsh et al., 1996 [107] | Multimodality therapy versus surgery | 3-year survival 32 % |
Transthoracic versus transhiatal approach | 5-year survival 36 % | |
Van Hagen, 2013 [17] | Multimodality therapy versus surgery | 5-year survival 47 % |
Table 4.2
Performance indicators that have been identified in esophageal cancer surgery
Quality-of-care indicators |
|---|
Structural measures |
Hospital volume |
Surgeon volume |
Centralization |
Process measures |
Discussion in multidisciplinary board |
Age |
Preoperative quality of life |
Staging (FDG-PET vs. FDG-PET) |
Lymphadenectomy |
Neoadjuvant chemoradiation |
Surgical approach |
Outcome measures |
Postoperative complications |
Radicality of resection |
Number of resected lymph nodes |
Finally, comparing the quality of infrequent operations such as esophagectomies is difficult, besides issues of definition and case-mix correction, because of another complex element in comparing surgical performance, i.e., the problem of sample size [115].
Conclusion/Take Home Messages
Discussion of all patients with esophageal malignancies in a multidisciplinary tumor board is recommended and is associated with improved outcomes after surgery.
ASA, (O)-POSSUM, and Charlson are the preoperative risk scoring systems that are often used in esophageal surgery.
The most important change in the most recent 7th edition of the TNM staging system is that the concept of non-regional lymph nodes has been abandoned and that staging of esophageal cancer has been harmonized with gastric cancer.
After extensive “conventional” diagnostic work-up, additional PET scanning yields a diagnosis of distant dissemination in an additional 10 % of patients, especially in case of T3 tumors.
The goals that have been achieved in open esophageal surgery should also act as targets for minimally invasive esophagectomy, being a lymph node retrieval of at least 15 nodes, R0 resection (>1 mm margin), and operative mortality < 5 %.
Neoadjuvant chemoradiotherapy decreases the incidence of a tumor-positive circumferential margin.
Meta-analysis of the available literature data did not show differences in survival between transhiatal and transthoracic operations. The survival benefit of an extended lymphadenectomy by a transthoracic approach seems to be limited to a subgroup of patients with low burden of nodal disease.
Overall, complication rates are reported in over 50 % of esophagectomy series, with incidences varying between 17 and 74 %. Postoperative complications have been directly linked to a variety of other outcome parameters including mortality, readmission rate, early cancer recurrence, survival, length of hospital stay, resource utilization, and quality of life.
It has been suggested that MIE is accompanied by less pulmonary complications.
The 30-day operative mortality (30DM) and the inhospital mortality (IHM) after esophageal resection vary from 4 % for specialized centers to > 10 % for nationwide registries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree