Method
Target
Sensitivity
Advantages
Disadvantages
Metaphase
t(9;22)
1–5 %
The gold standard and detection of other chromosomal changes
Needs dividing cells, so generally only successful form of bone marrow sample
Chromosome
Cytogenetics
FISH
BCR-ABL DNA
0.1–5 %
Can use peripheral blood or bone marrow
Relatively insensitive compared to RT-PCR
Quantitative
BCR-ABL mRNA
0.001–0.01 %
Very sensitive and uses peripheral blood
Not well standardized across labs
RT-PCR
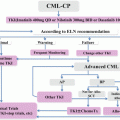
In general, treatment with TKIs can be viewed as a series of benchmarks that consider both the degree of reduction in disease markers, as well as the rapidity of response, both of which have clearly been shown to correlate with long-term outcomes in numerous clinical trials. Two nonprofit professional organizations, the US-based National Comprehensive Cancer Network (NCCN) and the European Leukemia Net (ELN), publish recommendations to guide clinicians in the best therapeutic and monitoring strategies for CML, with guidelines based on the most recent medical evidence. As of the time of publication, the 2015 NCCN and 2013 ELN guidelines represent the most recent consensus recommendations, the content of which are remarkably similar between the two groups [18, 19].
Once TKI treatment has begun, the first measure of response is hematological remission, which is simply the normalization of peripheral complete blood counts and normalization in spleen size. The term “complete hematologic response” (CHR) is specifically defined as normalization in peripheral blood counts (total leukocyte count of less than 10 × 109/L, platelet count of less than 450 × 109/L, and absence of circulating immature myeloid cells) and absence of splenomegaly (Table 4.2). The achievement of a CHR within 3 months of initiating therapy is widely recognized as a critical objective of modern therapy and is incorporated as the first milestone in guidelines from both the ELN and NCCN. For chronic-phase patients, this occurs in >90 % of patients within the first 3 months of therapy (and for the majority, sooner than that) [20]. ELN guidelines suggest the additional criterion of basophils comprising less than 5 % of the peripheral blood differential. Failure to achieve hematological remission, if the patient has been adherent to prescribed therapy, is an indication to change to another TKI.
Table 4.2
Response criteria in CML
Level of response | Definition |
|---|---|
Complete hematological response | Normal CBC and differential |
Minor cytogenetic response | 35–90 % Ph-positive metaphases |
Partial cytogenetic response | 1–34 % Ph-positive metaphases |
Complete cytogenetic response | 0 % Ph-positive metaphases |
Major molecular response | ≥3-log reduction of BCR-ABL mRNA; 0.1 % IS |
Complete molecular remission | Negativity by qPCR at a sensitivity of at least 4.5 logs (0.0032 % IS) |
Reduction in the amount of detectable Ph, as measured by conventional metaphase cytogenetics, represents the next stratum of response assessment. Cytogenetic responses are based on sequential bone marrow cytogenetic analyses, with evaluation of at least 20 metaphases required for optimal interpretation. A minor cytogenetic response (mCyR) and major cytogenetic response (MCyR) are defined by the presence of t(9;22) in 35–65 % and 1–35 % of metaphases, respectively. The more stringent definition of “complete cytogenetic response” (CCyR) indicates the absence of detectable Ph in at least 20 metaphases. For consistency between clinical trials, the standard approach to determining CCyR has been through conventional metaphase cytogenetics, and thus patient outcomes have been historically based on this. There is evidence to suggest that if such evaluation is not available, FISH <1 % (considering at least 200 cells) appears to be equivalent to CCyR [21] (Table 4.3).
Table 4.3
A comparison of “optimal” and “failure” criteria for NCCN and ELN guidelines
NCCN Guidelines®: version 1.2015 | 2013 European LeukemiaNet guidelinesd | ||||
|---|---|---|---|---|---|
Optimal | Failure (change of therapy advised) | Optimal | Warning | Failuree (change of therapy advised) | |
3 months | BCR-ABL ≤10 % (IS) or PCyR | BCR-ABL >10 % (IS) or lack of PCyRa,b | BCR-ABL ≤10 % (IS) or Ph+ ≤35 % | BCR-ABL >10 % (IS) and/or Ph+ 65–95 % | Lack CHR or Ph+ >95 % |
6 months | BCR-ABL ≤10 % (IS) or ≥ PCyR | BCR-ABL >10 % (IS) or lack of PCyR | BCR-ABL ≤1 % (IS) and/or CCyR | BCR-ABL 1–10 % (IS) and/or Ph+ 1–35 % | BCR-ABL >10 % (IS) and/or Ph+ >35 % |
12 months | CCyR or BCR-ABL ≤1 % (IS) | < PCyRa,c or BCR-ABL >10 % (IS) | BCR-ABL ≤0.1 % (IS) | BCR-ABL= 0.1–1 % (IS) | BCR-ABL >1 % (IS) and/or Ph+ >0 % |
18 months | CCyR | Less than CCyR or cytogenetic relapse | |||
Cytogenetic response continues to be the standard indicator of therapeutic success with TKI therapy. In the landmark IRIS trial, where patients were treated with imatinib versus interferon plus cytarabine, achievement of a CCyR at 6 months (on either treatment arm) was associated with a decreased risk of disease progression to advanced phase at a median of 42 months of follow-up [1]. Impact of CCyR on survival has also remained in patients receiving high-dose imatinib or second-generation TKIs [22]. Both the ELN and NCCN recognize the achievement of CCyR within a year of therapy as an extremely important milestone [18, 19].
The most sensitive, and convenient, method of measuring disease response during therapy is by BCR-ABL qPCR. With current methods, laboratories are able to detect as low as a single CML cell in a background of up to at least 100,000 normal cells. This high level of sensitivity allows routine disease monitoring to be performed on peripheral blood. BCR-ABL transcript levels determined by qRT-PCR are highly correlated with disease burden as determined by cytogenetics or FISH (at least, in the range that all three can be comparatively measured), and many centers with expertise in qRT-PCR use BCR-ABL transcript monitoring to monitor patients instead of cytogenetics, once cytogenetics have been performed at diagnosis to establish the diagnosis and stage of disease [23, 24].
There is no “standard” BCR-ABL qPCR assay, as the labs have variations in the platform used, primers, and even housekeeping genes that are used as controls. Much of what has been established for molecular monitoring stems from the landmark IRIS study (the phase 3 trial that established imatinib as the new standard for CML). In IRIS, a baseline BCR-ABL transcript level (measured as BCR-ABL/BCR) was determined through PCR testing of peripheral blood samples from 30 untreated, chronic-phase CML patients in each of the three IRIS laboratories [25]. Median values for the 30 samples served as the baseline BCR-ABL/BCR level for each laboratory, to which subsequent patient samples would be compared. The BCR-ABL log reduction value for each patient was calculated by comparing a result to the median value of the diagnostic reference group. A 3-log reduction from the median baseline was deemed a “major molecular response” (MMR), and this correlated with an excellent progression-free survival. Amazingly, other studies confirmed the importance of the MMR (see below), and MMR quickly became an important response metric [3, 5, 26, 27].
Unfortunately, the original specimen pool that was used to determine the baseline BCR-ABL/BCR transcript levels in the IRIS study has since been depleted. However, prior to the consumption of these specimens, an equivalent measure of BCR-ABL transcript levels was engineered, and thus a standard for BCR-ABL has been established, known as the International Scale [28]. Through exchange of samples with an IS reference laboratory, an “IS conversion factor” can be established for a particular lab, which will then allow for standardization of results to the IS. The IS has been conveniently aligned with important milestones for treatment, with a value of 1 % IS correlating with a complete cytogenetic response (CCyR) and an IS of 0.1 % indicating the level of MMR.
4.3 What Is the Best Definition of Response to Therapy in CML?
Molecular responses are defined using qRT-PCR for detection of BCR-ABL mRNA transcript levels. Fortunately, peripheral blood may be used for such monitoring by qRT-PCR. A major molecular response (MMR), a milestone that correlates with long-term prognosis (see below), is defined by a transcript level less than 0.1 %, or a greater than 3-log scale reduction, on the International Scale (IS). More stringent responses, such as “MR4” and “MR4.5” suggest molecular remission with undetectable transcripts at 4-log and 4.5-log scale reductions, respectively, from the IRIS baseline.
4.4 Treatment Milestones
There are several sequential treatment landmarks which are clearly associated with clinical outcome.
4.4.1 Complete Hematologic Response (CHR)
The complete normalization of counts generally occurs quickly, certainly within the first 3 months of therapy. Failure to reach a CHR obviously precludes a deeper cytogenetic and molecular response and triggers an immediate change of TKI therapy. In addition, failure to achieve a CHR should trigger suspicion about poor adherence to therapy.
4.4.2 Cytogenetic Response
Cytogenetic monitoring of the level of Ph+ metaphases is the next prognostic factor for predicting long-term response to TKI therapy once patients have achieved a CHR. Achieving a CCyR is an independent prognostic factor for survival and should therefore be considered a goal of therapy [1, 29, 30]. Failure to achieve any reduction in the number of Ph+ cells after 6 months of imatinib therapy and failure to achieve an MCyR response after 12 months of imatinib therapy predict for <20 % chance of ever achieving a CCyR [31]. Additionally, studies have shown that achieving an MCyR at 3 months is associated with prolonged time to disease progression in patients with late chronic-phase and accelerated-phase CML [32, 33].
4.4.3 Major Molecular Response (MMR)
While attaining a CCyR remains a major therapeutic milestone, the further achievement of MMR seems to be a “safe haven,” as secondary resistance and progression is relatively unusual once MMR has been achieved. In the IRIS trial, considering only those patients with a CCyR, there was a 97 % progression-free survival at 54 months in the subset with more than 3-log reduction (MMR), at 12 months, as opposed to an 89 % progression-free survival in the subset with less than an MMR at 12 months [34]. Additional studies have confirmed that achievement of MMR at either 12 or 18 months is associated with a longer duration of CCyR and higher rates of progression-free survival [35, 36]. Furthermore, loss of MMR after initially achieving one is associated with an increased risk of disease relapse [37].
4.4.4 Complete Molecular Remission (CMR)
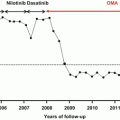
In vitro studies have demonstrated that the CML “stem cell” does not depend on BCR-ABL kinase activity for survival [38]. Given that finding, the prevailing assumption had been that patients would require TKI therapy forever. This is yet another example of popular assumptions being wrong. In fact, several studies have shown that for those (relatively rare) patients with persistently undetectable BCR-ABL, many patients may discontinue therapy without relapse of their disease, even by PCR criteria. The STIM trial and the Australasian Leukemia and Lymphoma Group’s CML8 TWISTER trial represent two landmark clinical trials investigating the clinical course of patients with undetectable disease by PCR testing [39, 40]. Both trials required that patients have undetectable BCR-ABL mRNA for at least 2 years to be considered eligible for TKI discontinuation, and in both a remarkably similar proportion of enrolled patients, 40 % maintained long-term remission after discontinuation of imatinib. Happily, those patients who did relapse responded to subsequent TKI therapy, though not all reverted back to an undetectable BCR-ABL transcript level.
In reality, the term “undetectable BCR-ABL” is complicated, as BCR-ABL can become undetectable given (1) a very low BCR-ABL level despite employment of a sensitive test; (2) a higher level of disease burden, but an inadequate sample size; and (3) almost any BCR-ABL level with a poorly sensitive test. Obviously, only the first such scenario would satisfy clinical requirements to consider discontinuing therapy. Thus, the concept of a complete molecular remission (CMR) pertains to that degree of response in which BCR-ABL is undetectable by PCR methods, backed with the potentially depth that the assay is able to reach given the number of cells tested. The copy number of the control gene estimates the cell numbers. Thus, CMR 4.5 would refer to undetectable disease in a sample adequate to assess a 4.5-log reduction in BCR-ABL level. Ongoing discontinuation studies differ by the depth of response required to consider discontinuation, but generally require at least a 4-log read depth. For example, the PCR sensitivity criteria for the above-referenced CML8 and STIM trials were set at a 4.5-log and 5-log reduction, respectively, from the IRIS baseline [39, 40]. It reasons that earlier and deeper molecular responses attained with second-generation TKIs might allow more patients to successfully discontinue TKI therapy, and prospective trials to address this issue are ongoing (Fig. 4.1).
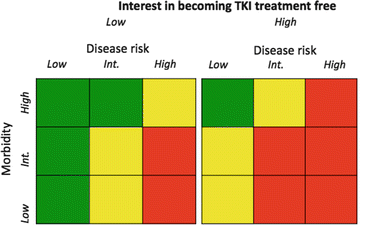

Fig. 4.1




An imaginary treatment grid based on morbidity, disease risk (Sokal), and desire to obtain a sustained CMR and successfully discontinue therapy. The colors are a heat map, where green is a strong choice toward imatinib, yellow intermediate choice between imatinib and a second-generation TKI, and red a strong preference toward a second-generation TKI
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree