html xmlns=”http://www.w3.org/1999/xhtml” xmlns:mml=”http://www.w3.org/1998/Math/MathML” xmlns:epub=”http://www.idpf.org/2007/ops”>
Précis
Definition of terms
Extent of resection: estimated volume of remaining tumor tissue present on postoperative MRI as compared to preoperative MRI
Low-grade glioma: WHO grade I and II lesions
High-grade glioma: WHO grade III and IV lesions
Recurrent GBM: WHO grade IV lesion that recurs after initial operative resection
T1/T2-weighted images: specific pulse sequences on MRI that result in different imaging signatures. Tissues will have a spectrum of appearances on T1- and T2-weighted images. As a generalization, tissues with high water content will be bright on T2, and fat and gadolinium-based contrast agents will be bright on T1.
Intraoperative electrical stimulation: technique used to guide surgical resection in areas of eloquent cortex
Laser-induced thermal therapy: method of using low-powered laser to generate heat in a controlled fashion to eradicate tumor tissue
Transcranial magnetic stimulation: non-invasive technique to stimulate the brain through an intact skull to define regions of eloquent cortex.
fMRI: an imaging technique which can be useful in helping to define regions of eloquent cortex by having the patient perform a preset group of tasks
DTI: imaging technique useful for delineating key white-matter tracts and their relationship to gliomas
ALA: intraoperative fluorescence technique to differentiate tumor tissue from normal surrounding brain.
Key points to be covered
Extent of resection is a critical marker for overall longevity and progression to higher-grade lesions in patients harboring both low- and high-grade gliomas. Greater extent of resection is also critical in extending the survival of patients with recurrent GBM.
Surgeon understanding of cortical and subcortical anatomy is key for achieving safe and complete resection of gliomas. Common anatomical variants must be recognized intraoperatively to avoid complications from damage to critical anatomical structures.
As with any surgical intervention, patient selection, recognition of medical comorbidities, and clear communication with anesthesia staff is paramount to safely operating on patients with gliomas.
Awake craniotomies are highly beneficial in maximizing extent of resection and safely operating around critical cortical and subcortical structures. Total intravenous anesthesia has made these procedures more efficient. Intraoperative electrical stimulation is vital whether the patient is asleep or awake during surgery.
Emerging techniques, including laser-induced thermal therapy, transcranial magnetic stimulation, functional MRI, diffusion-weighted tractography, and intraoperative aminolevulinic acid fluorescence are becoming more and more useful to aid neurosurgeons treating patients with gliomas.
Introduction
Historical background
The first successful brain tumor surgery was completed in 1879 by Dr. William Macewen. His work was expanded upon by surgical pioneers such as Horsley, Keen, Cushing, Penfield, Dandy, Ojemann, and many others and serves as the foundation for our current knowledge on brain tumor resection. Today, awake craniotomies with intraoperative mapping aided by complex navigational systems make brain tumor surgeries not only safe, but also effective. While other tumors are very responsive to surgical treatment, glioma management still remains one of the most challenging areas of neurosurgical practice. In the literature, evidence is continually revealed that extent of surgical resection in patients harboring gliomas plays a role not only in overall patient survival, but also in functional status.
Evidence-based practice
Extent of resection
Low-grade glioma
The literature indicates that extent of resection is not only a predictor of longevity, but also delays time to anaplastic transformation following low-grade glioma surgery. The question that is not explicitly answered in these sources, however, is whether removing more tumor improves survival in any given patient. To take this question one step further, does a greater extent of resection (EOR) indicate the tumor was less initially aggresive with an intrinsically better prognosis? Is EOR only a surrogate for a less invasive tumor? Gliomas can be compact or diffuse in nature. The more compact tumors are viewed as less aggressive or invasive in behavior. The glioma literature indirectly supports that greater EOR does increase longevity regardless of this inherent variability in intrinsic tumor biology and aggressiveness.
When attempting to define EOR, one major confounder is the method used to measure tumor volume from magnetic resonance imaging (MRI). The greatest diameter in one, two, or three axes has been used as an estimate of tumor size. Three-dimensional volumetric measurement is the current standard of tumor size estimation. In this method, the edge of the tumor is outlined on each imaging slice, and the volume is then calculated by integration of the stacked slices. This method is in and of itself an estimation. Below we review several key studies on EOR in low-grade gliomas using a variety of methods to estimate surgical success.
Prior to the widespread use of MRI images as postoperative instruments to measure EOR, several studies relied upon a surgeon’s clinical impression of EOR. Johannesen et al. reported on a retrospective review of 993 patients operated between 1970 and 1993 for a supratentorial low-grade glioma. Patients in the first part of this study did not have the benefit of computed tomography (CT) or MRI scanning, and thus, the operating surgeon estimated EOR. While the median survival increased in patients undergoing a complete resection, the improvement was not statistically significant.1 To add to this literature base, Phillipon et al. (1993) reported on 179 patients who underwent resection of a supratentorial grade I or II tumor2 EOR was determined primarily by retrospective review of operative reports. Resection was considered total in 25% of cases, subtotal in 53%, and biopsy in 22%. Overall survival (OS) was improved with greater EOR based on surgeon report.
As MRI has become more routinely available to glioma surgeons, the method of retrospectively determining EOR has also become more quantitative. Capelle et al. reviewed outcomes of surgical intervention in 1,091 patients with grade II gliomas.3 Tumor size was estimated using by using a three-dimensional ellipsor approximation. Resections were graded as 100% (n = 80), 50–99% (n = 418), and <50% (n = 431). There was a statistically significant negative correlation between volume of residual tumor and survival. In this study, 15-year OS was 50%, with 40% of patients going on to malignant tumor transformation at 11.5 years following initial diagnosis.
Smith et al. used a volumetric analysis of fluid-attenuated inversion recovery (FLAIR) MRI in 216 patients who had undergone surgical resection for low-grade gliomas.4 This study, albeit retrospective, controlled for Karnofsky performance score (KPS), histology, age, and location. OS was predicted by EOR, log of the tumor volume, and postoperative tumor volume. The findings of this study suggest that EOR greater than 80% is a statistically significant predictor of survival.
Ius et al. respectively analyzed 190 patients who underwent resection of low-grade gliomas using manually segmented reconstructions of three-dimensional volumes.5 They found that OS was a function of EOR, age, histology type, and congruence between the T1- and T2-weighted MRI images (DVT2T1). They found that tumor resection of at least 90% was a predictor of improved OS, progression-free survival (PFS), and less malignant transformation. Similar improvement was noted when there was less than 10 cm3 of tumor on the postoperative MRI scan. Ius et al. reviewed the effects of T1T2 congruency on OS, PFS, and malignant-free PFS. Both EOR and T1T2 congruency were independently associated with better outcomes. This finding indirectly indicates that a greater EOR results in an extended survival independent of the tumor’s biology.
McGirt et al. reviewed 180 patients who had undergone surgery for a low-grade glioma. Degree of resection was determined by a retrospective review of the postoperative radiology reports, relying on the FLAIR sequence. Only gross total resection predicted a statistically significant improvement of overall survival and PFS.6 Nitta et al.7 reviewed the EOR in 153 patients harboring low-grade gliomas determined from pre- and postoperative MRI scan (exact method not noted). Greater than 90% tumor resection was associated with a prolonged overall survival and PFS. Skrap et al., reporting on a series of 66 insular tumors, noted an increase in the rate of 5-year OS when more than 90% of the tumor was resected.8Ahmadi et al. retrospectively reviewed 130 patients with histologically confirmed low-grade tumor.9 They reported an impressive total resection rate of 70%. In these cases, a statistically significant benefit of gross total resection was noted for OS, but not PFS.
Finally, a unqiue study by Yordanova et al. looked at awake surgery on patients with tumors in non-eloquent brain tissue. In 15 patients, all tissue surrounding the tumors was removed until the surgeon reached cortical and subcortical areas critical for brain function.10 When compared to a matched group who underwent gross total resection, the group undergoing “supratotal” resection experienced fewer anaplastic tranformations.
The above-listed studies are all retrospective studies, which are inherently troubled by recall and confounder bias. Therefore, it is prudent to consider those that collect data in a prospective manner, especially with regard to EOR in low-grade gliomas. Several prospective studies, which we detail below, demonstrate prolonged survival to be associated with a greater EOR.
In a prospective cohort study, Jakola et al. reported on two exclusive providers for low-grade glioma therapy in adjacent regions of Norway.11 One provider favored performing diagnostic biopsies and expectant management, while the second provider favored early resection. Median and estimated 5-year survival was significantly better with aggressive resection at the time of diagnosis. In another study, where intraoperative MRI was used to improve EOR, Claus et al.12 measured tumor volume on pre- and postoperative spoiled gradient recall echo (SPGR) or T2-weighted images. They found that OS was significantly better in patients having undergone complete resection when compared to those with only partial tumor resection.
In prospective randomized studies investigating effects of radiation therapy, EOR is of prognostic value. Shaw et al., in a randomized study of patients treated with high- or low-dose radiation, found that gross total resection resulted in a statistically significant improvement in 5-year survival.13 Yeh et al. reported on 93 patients who underwent surgery for low-grade supratentorial gliomas to determine the effect of postoperative radiation therapy. EOR was determined by surgeon’s report and postoperative CT scan. Once again, total resection was associated with an improved 5-year OS.14 In a prospective randomized trial by the European Organization for Research and Treatment of Cancer, radiation dose was analyzed in combination with the histopathologic diagnosis of low-grade glioma.15 Patients were stratified as <50% resection (45%), 50–85% resection (30%), and 90–100% resection (25%), as determined by postoperative imaging and the surgeon’s clinical impression. Percentage of tumor resected had a positive correlation with survival on both univariate and multivariate analysis.
High-grade glioma
The natural history of malignant gliomas is significantly worse than that of low-grade gliomas. While the literature strongly supports an improvement in prognosis after gross total resection, the exact prognostic efficacy remains elusive given the lack of class-one evidence. As with low-grade tumors, the criteria for EOR reported in the literature for high-grade tumors has evolved with time. The older technique of determining EOR based on surgeon’s impression has been shown to be far less accurate than imaging studies in comparative studies. The modern standard is to evaluate EOR based on quantitative volumetric measurements. An additional caveat which surgeons reviewing EOR literature should be aware of is that older studies are more likely to mix tumor grades and histology. Thus grade III and IV tumors, such as anaplastic oligodendroglioma and anaplastic astrocytoma, may be considered in a parallel fashion, rather than as their own entities. This is of importance as the EOR is less a predictor of survival than of residual volume in anaplastic tumors.
A review of the literature raises the question as to what is the more important predictor of OS: EOR or volume of residual tumor?16 Most retrospective reviews found that EOR is a predictor of prolonged survival. An example of this is the review of 451 primary glioblastoma multiforme (GBM) resections by McGirt et al.6 EOR was based on the postoperative MRI radiology report. Adjusting for variables known to affect OS, gross total resection prolonged survival when compared to near-total resection. Both gross and near-total resection improved survival compared to subtotal resection. An additional retrospective review by Oszvald et al. compared the effects of EOR in patients greater than or less than 65 years of age. They found that patients over the age of 65 were less likely to be offered a resection, but for those who did undergo surgery, EOR predicted the same OS regardless of age.17
While there are no randomized prospective trials on the role of EOR, there are a few prospective observational studies in the literature. Brown et al. reported on 220 patients enrolled into a quality-of-life study.18 They found that patients with total resections had a 4-month increase in survival and an improved quality of life. Schneider et al. reported on 27 patients chosen prospectively to assess the value of intraoperative MRI scan.19 Ten patients who had a complete resection of their tumor experienced an increase in average survival from 237 to 537 days. Stummer et al. reanalyzed data from their randomized study of 243 patients who underwent the use of aminolevulinic acid (ALA) in brain tumor resection.20 Patients were divided into complete resection and incomplete resection, as demonstrated by postoperative MRI. Adjustments were made to compensate for biases of measured parameters between the two groups. Complete resection was associated with a 5-month increase in OS. This improvement in survival was maintained following adjustment for group biases. The author noted that increased resection did not compromise quality of life. Buckner et al. randomized 275 patients who had received standard-of-care therapy with either additional carmustine or bis-chloroethylnitrosourea (BCNU) or interferon.21 Using Cox multivariate regression models, they found that EOR was a significant determinate of survival. Ushio et al. reviewed the effects of tumor removal on quality of life in 105 patients harboring GBMs.22 Gross total resection as determined by MRI was associated with prolonged survival. Stark et al.23 reviewed 267 patients treated for GBM. Gross total resection as determined by postoperative enhanced CT was associated with longer survival.
Most series since 2000 have shown a significant correlation between improved long-term survival and gross total resection, but there are exceptions. Levin et al. reported on a mixed group of anaplastic oligodendrogliomas and anaplastic astrocytomas treated with accelerated fractionated radiotherapy and procarbazine, lomustine, and vincristine (PCV) chemotherapy.24 Stratifying surgical intervention into gross total resection, subtotal resection, and biopsy demonstrated prolonged survival with greater resection, but this did not reach statistical significance (P = 0.058). Keles et al. reported on the correlation of EOR and residual tumor volume in 102 patients harboring anaplastic astrocytomas.25 This study used volumetric measurements of the lesions depicted by T1 gadolinium-enhanced scans and T2-weighted scans. The authors found that survival did not correlate with EOR, but did correlate with the amount of residual abnormal signal on the postoperative MRI. Tortosa et al. looked at CT scans of 95 patients treated for anaplastic gliomas.26 In the absence of enhancement, surgical opinion was used to determine the degree of resection. Multivariable analysis did not show EOR to be a predictor of survival.
In the most recent literature, studies are using volumetric techniques for measuring EOR. Lacroix et al.,27 in a widely quoted paper, analyzed the results of GBM resection in 416 consecutive patients using volumetric techniques to characterize the pre- and postoperative MRI scans. A total of 233 of these patients had no prior therapy. Using minimal probability value methods, the authors found that EOR was significant in predicting improved OS at values greater than 98%. Sanai et al. retrospectively reviewed 500 consecutive patients operated on for GBM who had pre- and postoperative three-dimensional (3D) volumetric measurements of the tumors.28 The authors found a statistically significant improvement in OS when more than 78% of the preoperative tumor was removed. The improvement in survival was most impressive at higher EORs. Chaichana et al. employed volumetric analysis to assess EOR in 84 patients who were thought to be good candidates for gross total resection on preoperative assessment.29 They found that a residual volume of less than 2 cm3 and EOR greater than 95% were independently associated with the greatest reduction in the hazards of death.
Recurrent GBM
The literature we have reviewed to this point addressed surgical treatment of primary gliomas. One critical question not answered by these prior studies pertains to the role of surgery in extending quality longevity with tumor recurrence. This question has been addressed in a number of retrospective cohort studies published after 1998. The majority of these studies suggest that re-resection at the time of recurrence can portend better survival.
Barker et al. published a review of 176 patients, 46 of which underwent a second surgery at the time of recurrence.30 They showed improved survival of 36 weeks versus 23 weeks in those undergoing re-resection. However, 23% of patients had a worsening of their KPS score following revision surgery. Helseth et al.31 reported an increase in survival from 8.6 to 18.4 months in 65 patients who underwent re-resection, compared with 65 patients treated non-operatively. Mandl et al.32 reported on a small number of patients who chose amongst surgery, chemotherapy, stereotactic radiosurgery, or stereotactic radiosurgery plus chemotherapy. The surgical patients trended toward prolonged survival, though this outcome did not reach statistical significance. A recent review of the 31 studies since 1980 demonstrated prolonged survival or improved functional states following re-resection of a malignant glioma 94% of the time.
Greater chance for improved survival also correlates with greater EOR. Bloch et al. reviewed 107 patients who underwent re-resection following GBM recurrence.33 Using the Cox proportional hazard model, patients with a greater than 95% resection had a longer OS than patients with less than 95% resection. Oppenlander et al.34 reviewed 270 patients undergoing secondary tumor resection. Using similar statistical analysis, they found that length of survival was predicted by EOR. As little as a 78% resection was associated with an improved survival.
The aforementioned studies suggest that the majority of patients with recurrent GBM should undergo secondary surgical resection. However, when appraising the literature and attempting to apply the results of these retrospective reviews, neurosurgeons must interpret these findings with care. Inherent selection bias comes to the forefront in patients chosen for a re-resection. These patients are typically more optimal surgical candidates, functioning at a higher level, with less comorbidities. Their tumor anatomy is commonly more favorable, portending to higher EOR and thus overall longevity. Therefore, these studies, although optimistic, are laden with confounders affecting the applicability and generalizability of re-resection of recurrent GBM. One reason for these results again returns to the fact noted just above: patients faring better with their disease are more likely to undergo multiple surgeries.
Multiple studies favor re-resection; however, there are those that suggest against additional surgical intervention for GBM. In 2011, the North American Brain Tumor Consortium reported on the prognostication of surgery at the time of progression.35 A second surgery was not a predictor of improved OS or 6-month PFS. Park et al. followed, publishing a scale to predict survival following secondary resection of a glioblastoma.36 They found that tumors with volumes greater than 50 cm3, KPS of 80 or less, and involvement of eloquent areas of brain significantly predicted a poor survival. Thus, secondary surgical intervention for GBM recurrence is not always favorable.
Anatomy: cortex
Knowledge of the anatomy of the brain is essential to surgical planning. Cortical sulci frequently define the limits of the gross tumor mass. While sulcal patterns vary from patient to patient, knowledge of the most common sulcal patterns alerts the surgeon to locations known to have gaps in the sulcal walls. Incomplete knowledge of these breaks can unexpectedly lead a subpial dissection into adjacent gyri and potentially eloquent brain. Although function does not necessarily follow a uniform anatomy, the surgeon should also be aware of the predominant cortical areas and subcortical pathways involved in language, motor, and visual function.
Frontal-lobe sulcal anatomy knowledge will aid dominant-hemisphere operation. The middle frontal gyrus is frequently divided longitudinally by the intermediate sulcus. The surgeon should not confuse the intermediate sulcus with the superior or inferior frontal sulcus. The inferior frontal sulcus is often interrupted, so the surgeon performing a subpial tumor dissection in the middle frontal gyrus may inadvertently wander into the inferior frontal gyrus. A break in the inferior frontal sulcus over the pars triangularis allows the surgeon removing a middle frontal lesion to wander into Broca’s area. The pars opercularis may be divided by an accessory sulcus or fuse with the posterior limb of the pars opercularis. Similarly, the precentral sulcus is usually interrupted at the level of the middle frontal gyrus. Thus, a subpial dissection along the precentral sulcus may inadvertently lead the surgeon into the motor strip. The pars orbitalis connects with the lateral orbital gyrus. This connection occurs anterior to the insula so that operating through these gyri provides an ideal pathway to anterior insular tumors. Additionally, there is usually a prominent subarachnoid space below the tip of the pars triangularis, providing a passage into the sylvian fissure.
The position of an inferior frontal language area (Broca’s area) varies among patients. Using a magnetic transcranial stimulator, Rogic et al.37 found that Broca’s area lay 17.2 mm (range 12.5–26.5 mm) anterior to the motor strip. Using intraoperative electrical stimulation and number counting, Quiñones-Hinojosa et al. reported Broca’s area to lie 1.89 ± 0. 98 cm above the sylvian fissure and 1.44 ± 0.48 cm anterior to the central sulcus.38
There are no prominent sulci separating the pre- and postcentral gyri along the medial surface of the hemispheres. This paracentral lobule is delineated anteriorly by a paracentral sulcus and posteriorly by the marginal ramus of the cingulate sulcus. Gaps in the cingulate sulcus and variations in the paracentral sulcus and marginal limb of the cingulate sulcus have been noted. The central sulcus is interrupted above the sylvian fissure where the pre- and postcentral gyri merge. At the level of the superior frontal sulcus, the “knee” in the precentral gyrus marks the motor hand region.
The deep olfactory sulcus overlying the olfactory tract and bulb can be followed posterior to the anterior perforating substance. The perforating arteries traveling through this region limit the resection of inferior frontal tumors.
The boundaries of the parietal lobe are particularly poorly defined at the time of surgery. The parietal lobe is divided horizontally by a well-defined intraparietal sulcus, which runs from the postcentral sulcus to the occipital lobe. A breach of the superior end of the postcentral sulcus allows communication between the postcentral gyrus and the superior parietal lobule. Medially, the superior parietal lobule is contiguous with the precuneatus, and posteriorly, the superior parietal lobule is contiguous with the superior occipital gyrus. The precuneatus gyrus is well delineated by the marginal sulcus anteriorly and the deep parieto-occipital sulcus posteriorly. Inferiorly lies the subparietal sulcus. The precuneatus provides an optimal pathway for removing tumors posterior to the sensory fibers and anterior and medial to the visual fibers.
The supramarginal and angular gyri are U-shaped loops bent around the posterior ramus of the Sylvian fissure and the terminal superior temporal sulcus, respectively. As such, the posterior limb of the supramarginal gyrus communicates with the superior temporal gyrus, and the anterior and posterior limb of the angular gyrus may communicate with the superior and middle temporal gyri, respectively. The connection with the superior temporal gyrus is usually blocked by an ascending sulcus that separates the angular from the supramarginal gyrus, however. The posterior limb also communicates with the middle occipital gyrus. The surgeon operating in the inferior parietal lobe must be careful not to wander inferiorly into language-related temporal cortex or the medially located superior longitudinal fasciculus and arcuate fasciculus.
For orientation, the postcentral gyrus lies superior to the lateral end of Heschl’s gyrus, which separates the temporal operculum into the anterior planum polare and posterior planum temporale.39 The superior temporal sulcus is well defined but the inferior temporal sulcus has several breaks, and is therefore not a reliable barrier between the middle and inferior temporal gyri.
The superior temporal sulcus bifurcates posteriorly into an anterior limb, which separates the supramarginal from the angular gyri, and a horizontal limb, which is embedded in the angular gyrus. Thus, the superior and middle temporal gyri communicate with the supramarginal and angular gyri, respectively. The border between the lateral posterior temporal lobe and the lateral occipital lobe is not well defined, and the anatomy of the region is variable. The middle and inferior temporal gyri communicate with the occipital gyri.
The inferior temporal gyrus is the lateral boundary of the inferior surface of the temporal lobe. This is separated from the fusiform gyrus by the occipital temporal sulcus. On the medial side of the fusiform gyrus, the collateral sulcus separates the fusiform gyrus from the parahippocampal gyrus.40 Anteriorly and posteriorly, these two sulci join and delineate the fusiform gyrus. The collateral sulcus continues posteriorly, separating the lingual of the occipital lobe from the inferior occipital gyrus. It continues forward as the rhinal sulcus. These two sulci serve as landmarks for the surgeon removing a tumor from the temporal lobe. The collateral sulcus lies directly under the temporal and occipital horns of the lateral ventricle. As long as the surgeon resects below the crest of the collateral gyrus, the optic radiations and inferior fronto-occipital tracts remain safe.
The parahippocampal gyrus occupies the mesial corner of the basal temporal lobe and extends on to the inferior mesial temporal lobe. The parahippocampal gyrus is separated from the dentate gyrus by the hippocampal sulcus. The parahippocampal gyrus curves back on itself medially to the amygdala, forming the uncus. The floor of the temporal horn is formed laterally to medially by the laterally located collateral indentation of the hippocampus and the fornix. Medial to the fornix is the choroidal fissure. This is a key landmark during subpial dissections, with a “safe” dissection focused from lateral to medial, and inferior to the fissure. Operating superior to the fissure leads the surgeon into the optic radiation and the descending motor fibers. The surgeon is protected from wandering into the deep structures by the continuous medial pia below the choroidal fissure. Anterior to the termination of the choroidal fissure, when removing amygdala and uncus, the surgeon must respect the globus pallidus and thalamostriate branches of the middle cerebral artery.
The convexity of the occipital lobe is continuous with the temporal and parietal lobes. As highlighted above, there is a variable gyral pattern. The inferior occipital lobe is divided in the sagittal plane into the inferior occipital and lingual gyri by the collateral sulcus. The optic radiations, which cover the lateral wall of the atrium of the lateral ventricle, lie superior and lateral to the sulcus. The deep parietal occipital sulcus separates the precuneatus of the parietal lobe from the occipital lobe and is an excellent landmark at the time of surgery. The calcarine sulcus separates the medial surface of the occipital lobe into the superior cuneal and inferior lingual gyri. Medial to the parietal occipital fissure, the calcarine sulcus separates the parahippocampal and cingulate gyri.
Anatomy: white matter
In addition to cortical anatomy, surgeons must recognize the location of the underlying white-matter tracts. As the cortical spinal tract passes from the motor cortex to the posterior limb of the internal capsule, motor leg fibers move in an anterior direction. The corticospinal fibers lie under the sensory face cortex and at the top of the anterior long gyrus of the insula. Perforating vessels passing through the lower central sulcus and upper posterior insula supply the deep segment of this tract.
The superior longitudinal fasciculus I links the superior parietal lobule with the motor cortex.41 This fasciculus is important in coordinating movement. Interruption of these fibers can result in apraxia.
The subcallosal fasciculus or Muratoff’s bundle links the supplementary motor cortex with the caudate nucleus.41 Interruption of this fasciculus produces elements of a supplementary motor syndrome with a particular emphasis on diminished spontaneous speech. Stimulation results in motor aphasia. There is evidence to suggest the paucity of speech is due to lesions of the recently described aslant fasciculus, which connects the pre-supplementary motor area to the inferior frontal gyrus.
Superior longitudinal fasciculus II and III extend from the angular gyrus and supramarginal gyrus to the posterior frontal lobe, respectively. These fibers are thought to moderate phonemic and articulating language, and verbal memory. Stimulation of these fibers results in speech arrest, speech apraxia, and phonemic paraphasic errors and articulating disorders.
The arcuate fasciculus runs deep to and extends superiorly to the superior longitudinal fasciculus II, III. It bridges from the dorsolateral frontal lobe to the caudal temporal lobe. The arcuate fasciculus is found deep to the middle frontal gyrus and inferior parietal lobule, terminating in the posterior middle and superior temporal gyri. Stimulation of the fasciculus can produce phonemic paraphasias.
The optic radiations carrying visual fibers emanate from the lateral geniculus passing through the temporal stem. Fibers destined to the lingual gyrus travel forward over the superior temporal horn before doubling back over the lateral temporal horn. Fibers headed to the cuneatus pass over the atrium of the lateral ventricle. The fibers of the optic radiation pass below the occipitofrontal fasciculus.
The inferior occipitofrontal fasciculus connects the superior parietal lobe, all three gyri of the occipital lobe, and the basal temporal lobe with the inferior and lateral frontal lobe. The fibers of the occipitofrontal fasciculus travel superior and lateral to the optic radiations. These fibers, along with the arcuate fasciculus and superior longitudinal fasciculus II, complete the circuit mediating language function. These fibers travel over the temporal horn and through the temporal stem. Interruption of the inferior frontal fibers results in semantic paraphasic errors. Additionally, a fiber bundle traveling through the extreme capsule, connecting the middle and superior temporal gyri with the inferior frontal lobe, passing through the extreme capsule, has been described in monkeys. These fibers seem to be a continuation of the occipitofrontal fibers and are anatomically positioned to be involved with language function.42
The uncinated fasciculus connects the anterior temporal lobe with the orbital and polar segments of the frontal lobe. It passes through the temporal stem, anterior and ventral to the inferior occipitofrontal fasciculus. The uncinated fasciculus has been associated with semantic processing, episodic memory, and emotional processing. A recent theory proposes that the uncinated fasciculus conveys mnemonic associates to influence lateral frontal-lobe decision making.43 Most patients suffer little more than difficulty with retrieval of proper nouns after removal of the uncinated fasciculus and adjacent cortex.
Patient selection
Although removing greater than 80% of a tumor predicts prolonged survival, survival is extended most significantly when more than 95% of the tumor is removed.4,5,28,34 Except for the rare cases in which surgery is aimed at establishing a diagnosis or relieving mass effect, a neurosurgeon does not undertake surgery unless optimal tumor debulking is anticipated.
Imaging characteristics
Gliomas appear to have two patterns of growth, one displacing and the other infiltrating adjacent brain. Tumors, which predominantly displace adjacent brain, are most amenable to gross total surgical resection. Low-grade gliomas differ from high-grade lesions in appearance on imaging. Solid components are likely to be dark on T1-weighted MRI and bright on T2- or FLAIR-weighted sequences. In contrast, infiltrating components are more likely to be isodense with surrounding brain on T1-weighted sequences, but may be detected as bright on T2- or FLAIR-weighted images. The solid-component higher-grade tumors are seen as gadolinium-enhancing masses on T1-weighted MRI. The diffuse component of the tumor tends to be isodense on T1-weighted images and bright on T2- or FLAIR-weighted images. Surgeons often struggle to achieve a gross total resection when T2 hyperdense, T1 isodense tumors involve eloquent cortex or white-matter fibers.
Patient-specific factors
Prior to surgical intervention, a neurosurgeon must consider the patient’s general overall health, tumor history, and neurological status. Comorbid heart disease, malnutrition, pulmonary compromise, and connective tissue disorders all increase operative risk. However, age itself is not an absolute surgical contraindication. One must anticipate, though, that age is directly correlated with recovery time and medical postoperative complication. Patients with significant neurological deficits concomitant with a predominantly infiltrating tumor, and patients with marked altered mental status without significant mass effect, rarely improve with surgery.
Preoperative planning
Dexamethasone, the most commonly used corticosteroid in neurosurgery, has a long half-life and is generally given every 6 hours to reduce peritumoral swelling. Short-term administration can cause insomnia, increased appetite, hyperglycemia, leukocytosis, gastritis, peptic ulcers, and mild mania. Longer-term use is associated with Cushingoid habitus, suppression of the adrenal–hypothalamic system, hypertension, severe psychiatric disturbances, osteoporosis, myopathy, Pneumocystis pneumonia, and aseptic joint disease. While the efficacy of H2 blockers and proton pump inhibitors has not been proven to lower the incidence of gastrointestinal complications, they are typically prescribed in combination with dexamethasone.
No benefit of preoperative anticonvulsants has been shown in seizure-naïve patients. Some surgeons administer a short course of anticonvulsants in the postoperative period based on literature surrounding patients with head trauma. Anticonvulsants are commonly employed in the immediate postoperative period, up to 72 hours, as the majority of seizures occur in the first 72 hours following surgery.44 Long-term postoperative anticonvulsant therapy has not proven beneficial.45,46
Invasive and non-invasive surgical interventions for glioma
Craniotomy
Prior to the surgery, the surgeon should have a detailed plan of attack. If the patient is to remain asleep throughout the entire procedure, the patient should be positioned so that the surgeon is directly facing the tumor. This is important for those tumors that breach the cortical surface, fall in line with the planned trajectory, or lie deep in the brain. A neurosurgeon is more comfortable and effective operating parallel to the plane of vision, rather than perpendicular, so as to limit the degree of tangential movements.
If the patient is to be awakened during tumor resection, attention must be awarded to making sure the patient is comfortable. The patient should be in a neutral position, whether supine or lateral, and the operative table is moved to accommodate the surgeon’s planned trajectory. All pressure points must be carefully padded. The patient’s neck should be as close to neutral as possible, as neck fatigue is a frequent intraoperative patient complaint. The Foley catheter is a frequent cause of irritation and should be coated with lidocaine jelly prior to insertion. Surgical drapes should be widely spread to avoid inducing patient claustrophobia and anxiety. When done properly, with patient-centered care at the forefront, awake surgery is generally well tolerated. In Taylor and Bernstein’s series of 200 patients selected for awake craniotomy,47 98% were able to participate in the procedure. As a bonus, these patients had shorter than expected hospital stays. Most retrospective reviews indicate that patients undergoing electrical brain mapping awake during surgery have fewer postoperative neurological deficits and experience a greater EOR than patients who are not woken intraoperatively.
After the induction of anupesthesia and positioning, attention is turned toward starting the craniotomy. Schools of thought regarding the size of the craniotomy flap vary considerably. Some recommend large flaps, especially for awake patients, allowing for more comprehensive mapping of neurological function. Others employ smaller openings, removing the tumor from the inside out. We tailor the flap to expose the entire cortical surface over the tumor. When mapping neurological function, we slide a recording electrode under the dura to stimulate function not directly adjacent to the tumor. Whenever possible we stimulate cortical movement with this subdural electrode. Once we have proven our mapping system is functioning, we confidently interrogate the cortex to be removed, mapping for positive and negative responses.
For most tumors, defining the plane between the gross and the adjacent brain is the next key step. When a tumor does not encroach on eloquent cortex or white-matter tracts, an attempt to circumscribe the tumor and remove it en bloc is made. By following the plane between normal- and abnormal-appearing tissue, one is more likely to achieve a gross total resection. Tumors abutting eloquent brain are grossly debulked to decrease the overall mass and the positions of tumor not adjacent to eloquent brain are removed. Attention is then returned to the interface between tumor and eloquent cortex or white matter where the tumor is shaved stimulating the tissue ahead of the dissection, mapping for eloquent function. If the electrical stimulation is negative, the tumor is continually shaved until normal-appearing brain is encountered.
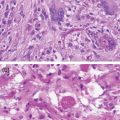
The consistency and appearance of tumors vary tremendously. Malignant gliomas often have a firm consistency and appear to be limited by cortical sulci. Portions of the tumor may be relatively soft and slightly more gray and purple than the surrounding parenchyma. As the tumor is manipulated, it often becomes edematous, purple, and hemorrhagic. This change in appearance clouds border definition, highlighting the importance of defining and mapping boundaries prior to significant tumor manipulation. Occasionally, malignant tumors will differ only slightly from the normal brain, being slightly tan, a little more firm, and punctated with small thrombosed blood vessels.
Low-grade tumors also vary considerably in their gross appearance. The best defined of these tumors are softer and are a darker gray than surrounding brain. The tumor tissue often produces a significant number of bubbles when cauterized with bipolar cautery. Infiltrating tumors are typically more viscous and firm than normal white matter, while other tumors are waxy and firm. The latter are best removed with an ultrasonic aspirator due to their hard consistency. Once the surgeon begins working in a low-grade tumor, he or she will note that particular tumor’s characteristics, and should stop resection once reaching tissue of a more normal consistency and appearance.
Anesthesia
A detailed discussion of neuroanesthesia is beyond the scope of this chapter. As a number of agents can be employed to obtain general anesthesia, it is important that the surgeon communicate the goals and anticipated challenges of the procedure with the anesthesia team prior to the start of surgery. The anesthesiologist should be aware of the patient’s systemic risk factors and reactions to prior anesthetics. Anesthetic agents vary in their effect on cerebral blood flow, cerebral blood volume, cerebral metabolism, and coupling of cerebral blood flow to cerebral metabolism. These factors affect not only intraoperative events, but also the rapidity of emergence from anesthesia, and therefore, should be tailored to the patient’s hemodynamic and intracranial pressure status.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree